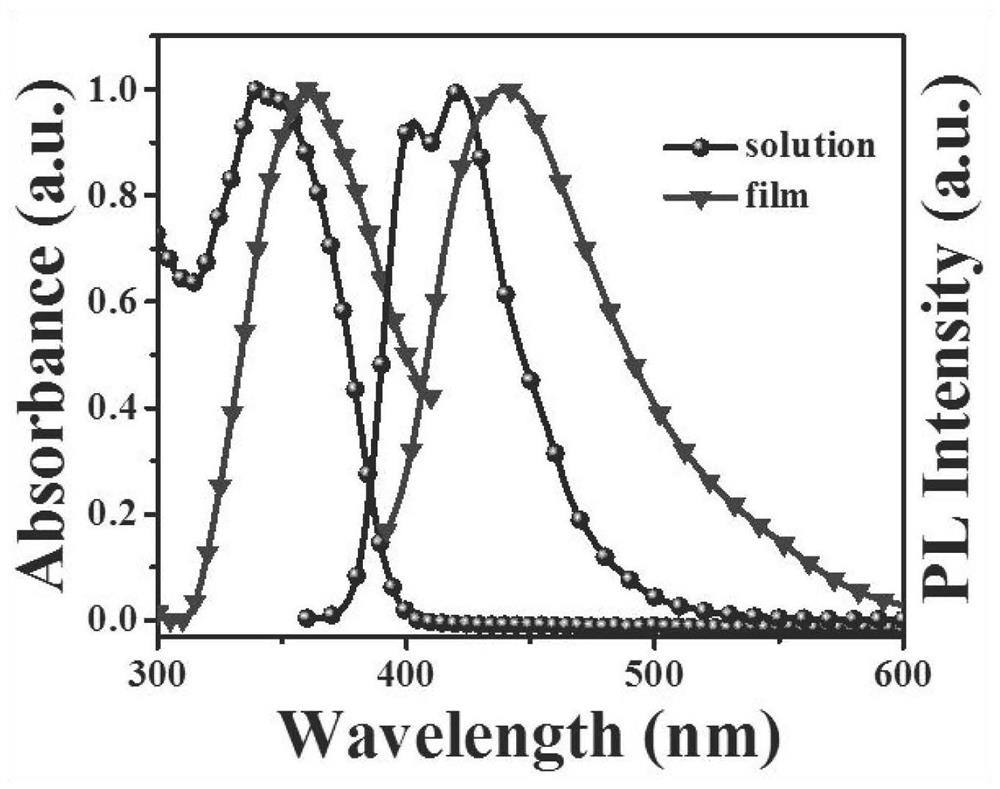
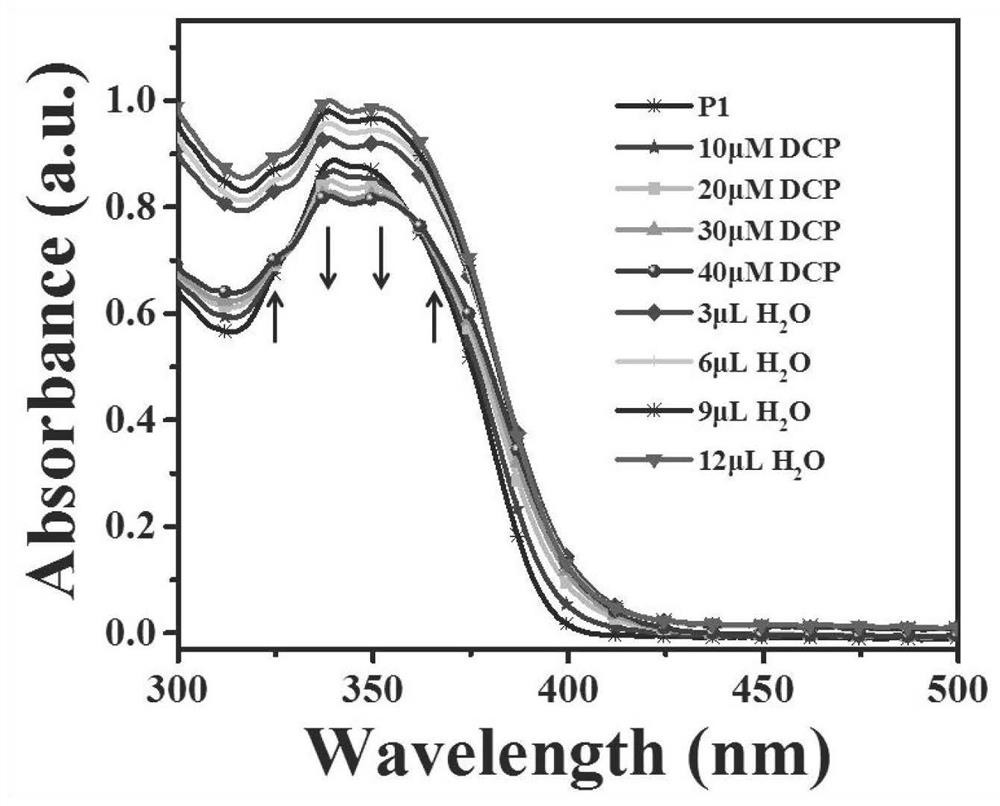
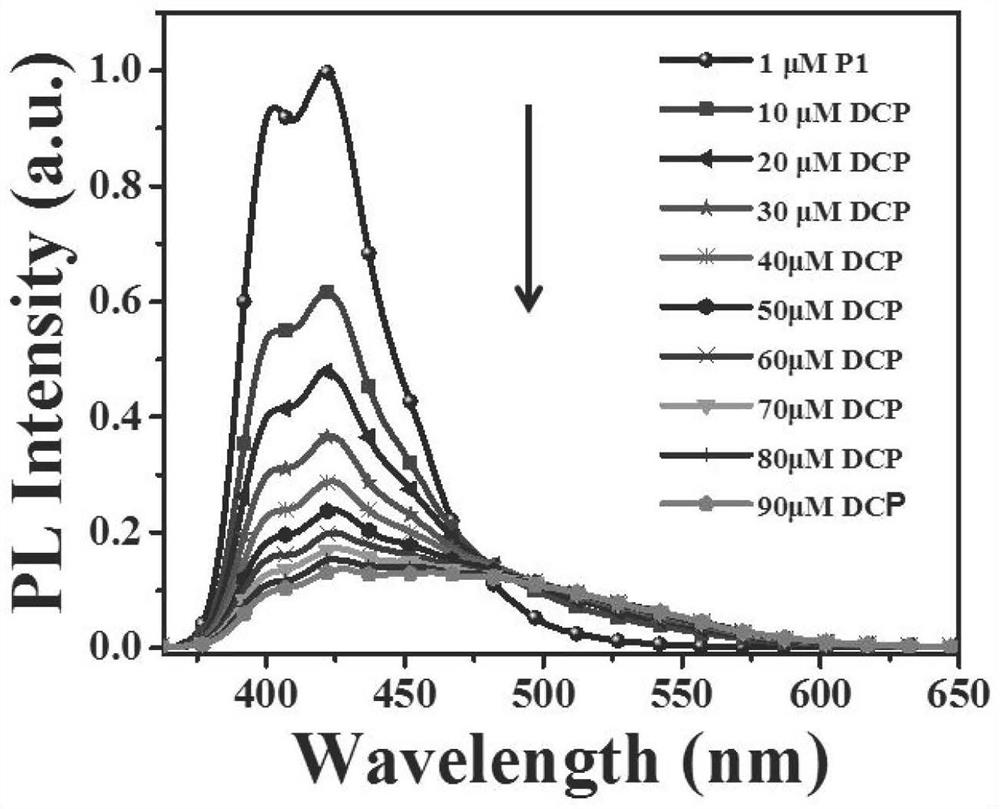
Sensors and their applications, standard fluorescent cards
A sensor and fluorescence sensor technology, applied in the field of fluorescence sensing, can solve the problems of slow response speed of detection materials, inability to use gas phase detection, poor repeatability, etc., and achieve the effects of good electron transmission capability, strong rigidity, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The present invention provides a preparation method for the fluorescent polymer described in the above scheme, comprising the following steps: performing a coupling reaction with the boronic acid corresponding to the structural unit A or the boric acid ester corresponding to the structural unit A with a compound having a structure shown in formula II, A fluorescent polymer having a structure shown in formula I is obtained;
[0059]
[0060] In the formula II, X is chlorine, bromine or iodine.
[0061] Unless otherwise specified, the raw materials used in the present invention are commercially available products well known in the art.
[0062] In the present invention, the boric acid ester corresponding to the structural unit A preferably has the structure shown in formula III:
[0063]
[0064] In the present invention, the structural formula of the boronic acid corresponding to the structural unit A is shown in formula IV:
[0065]
[0066] In the present in...
Embodiment 1
[0099] The preparation process of fluorescent polymer P1 is as follows:
[0100]
[0101] Specific steps include:
[0102] 150mg (0.24mmol) 1,2-two ((4-bromophenyl) chloromethylene) hydrazine, 142mg (0.24mmol) N-dodecyl-2,7-dioxaborocarbazole , 3mL of toluene, 2mL of potassium carbonate solution (concentration is 2M) and 1mL of absolute ethanol were mixed, under the protection of argon, the raw material mixture was frozen by liquid nitrogen for 8min, then vacuumized for 5min, and then 8.4mg of tetrakis(triphenylene) was added Phosphorus-based) palladium, repeat the above-mentioned freezing-vacuumizing operation three times, heat up to 100°C and reflux for 72h, after the reaction is completed, cool the resulting reaction solution to room temperature. The reaction solution was added dropwise into 200 mL of methanol, extracted by Soxhlet for 18 h and then dried to obtain the product with a calculated yield of 67%, which was designated as P1.
[0103] The obtained product P1 ...
Embodiment 2
[0107] The preparation process of fluorescent polymer P2 is as follows:
[0108]
[0109] Specific steps include:
[0110] 150mg (0.24mmol) of 1,2-bis((4-bromophenyl)chloromethylene)hydrazine, 142mg (0.24mmol) of 9,9 diethyl-2,7-dioxabororene, Mix 3mL of toluene, 2mL of potassium carbonate solution (concentration: 2M) and 1mL of absolute ethanol. Under the protection of argon, freeze the raw material mixture by liquid nitrogen for 8min, then vacuumize for 5min, and then add 8.4mg of tetrakis(triphenyl Phosphorus) palladium, repeat the above-mentioned freezing-vacuumizing operation three times, heat up to 100°C and reflux for 72h, after the reaction is completed, cool the resulting reaction solution to room temperature. The reaction solution was added dropwise into 200 mL of methanol, extracted by Soxhlet for 18 h and then dried to obtain the product with a calculated yield of 70%, which was designated as P2.
[0111] 1 H NMR (500MHz, CD 2 Cl 2 )δ8.24 (d, J = 7.4Hz, 4H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com