Preparation method of copper-zinc catalyst
A catalyst, copper-zinc technology, applied in the field of preparation of copper-zinc catalysts, can solve the problems affecting the physical and chemical properties of the catalyst, the dispersion effect of active components is not obvious, etc., and achieve the effect of high selectivity and heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
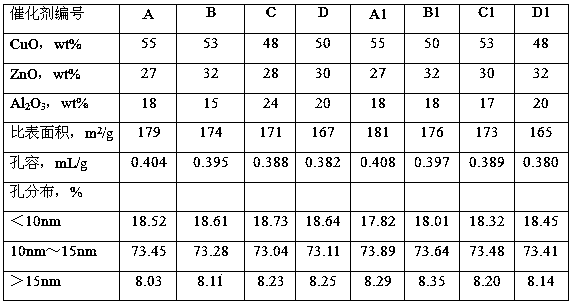
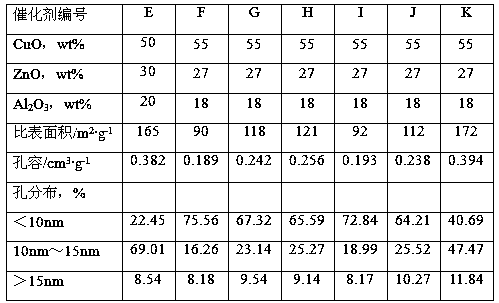
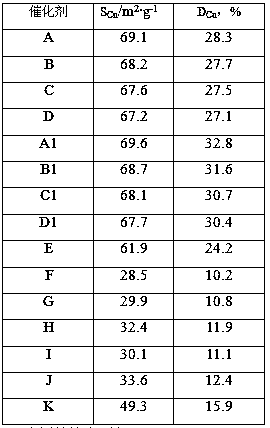
[0036] Cu(NO 3 ) 2 ·3H 2 O, Zn(NO 3 ) 2 ·6H 2 O was dissolved in deionized water, added 116 grams of hydroxyethylidene diphosphonic acid and mixed uniformly to prepare mixed solution A, Cu 2+ The concentration is 2.8mol / L, Zn 2+ The concentration is 2.5mol / L. Cu(NO 3 ) 2 ·3H 2 O and AlCl 3 ·6H 2 O was dissolved in deionized water to form a mixed solution B, Cu 2+ Concentration is 1.8mol / L, Al 3+ The concentration is 2.0mol / L. Add deionized water in the reaction tank, sodium metaaluminate solution (containing Al 2 o 3 42g / L) and the mixed solution A were added into the reaction tank concurrently, the gelling temperature was 60°C, the gelling pH was 7.5, and the gelling time was 0.9 hours to obtain slurry I. The slurry I was aged under stirring, the stirring speed was 190 rpm, the aging temperature was 75° C., the pH value was 7.2, and the aging time was 0.7 hours. After the aging is over, add the mixed solution B and the sodium carbonate solution into the aged ...
Embodiment 2
[0038] According to the method for embodiment 1, by the component content ratio of catalyst B in table 1, Cu(NO 3 ) 2 ·3H 2 O, Zn(NO 3 ) 2 ·6H 2 O was dissolved in deionized water, added 188 grams of ethylenediamine tetramethylene phosphonic acid and mixed uniformly, and mixed solution A was prepared, and Cu(NO 3 ) 2 ·3H 2 O and Al 2 (SO 4 ) 3 18H 2 O was dissolved in deionized water to form mixed solution B. Add deionized water in the reaction tank, sodium metaaluminate solution (containing Al 2 o 3 54g / L) and the mixed solution A were added into the reaction tank in parallel, the gelation temperature was 55°C, the pH value of the gelation was 7.0, and the gelation time was 0.8 hours to obtain the slurry I. The slurry I was aged under stirring, the stirring speed was 240 rpm, the aging temperature was 76° C., the aging pH value was 7.4, and the aging was 0.6 hours. After aging, the mixed solution B and sodium carbonate solution are added into the slurry I in par...
Embodiment 3
[0040] According to the method for embodiment 1, by the component content ratio of catalyst C in table 1, Cu(NO 3 ) 2 ·3H 2 O, Zn(NO 3 ) 2 ·6H 2 O was dissolved in deionized water, and 195 grams of polyacrylic acid (molecular weight: 3000) and 95 grams of hydroxyethylene diphosphonic acid were added and mixed evenly to prepare mixed solution A. Cu(NO 3 ) 2 ·3H 2 O and Al(NO 3 ) 3 9H 2 O was dissolved in deionized water to form mixed solution B. Add deionized water in the reaction tank, sodium metaaluminate solution (containing Al 2 o 335g / L) and mixed solution A were added into the reaction tank in parallel, the gelation temperature was 48°C, the pH value was 8.0, and the gelation time was 1.3 hours to obtain slurry I. The slurry I was aged under stirring, the stirring speed was 190 rpm, the aging temperature was 78° C., the aging pH value was 7.2, and the aging time was 0.5 hour. After the aging is finished, the mixed solution B and the sodium carbonate solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com