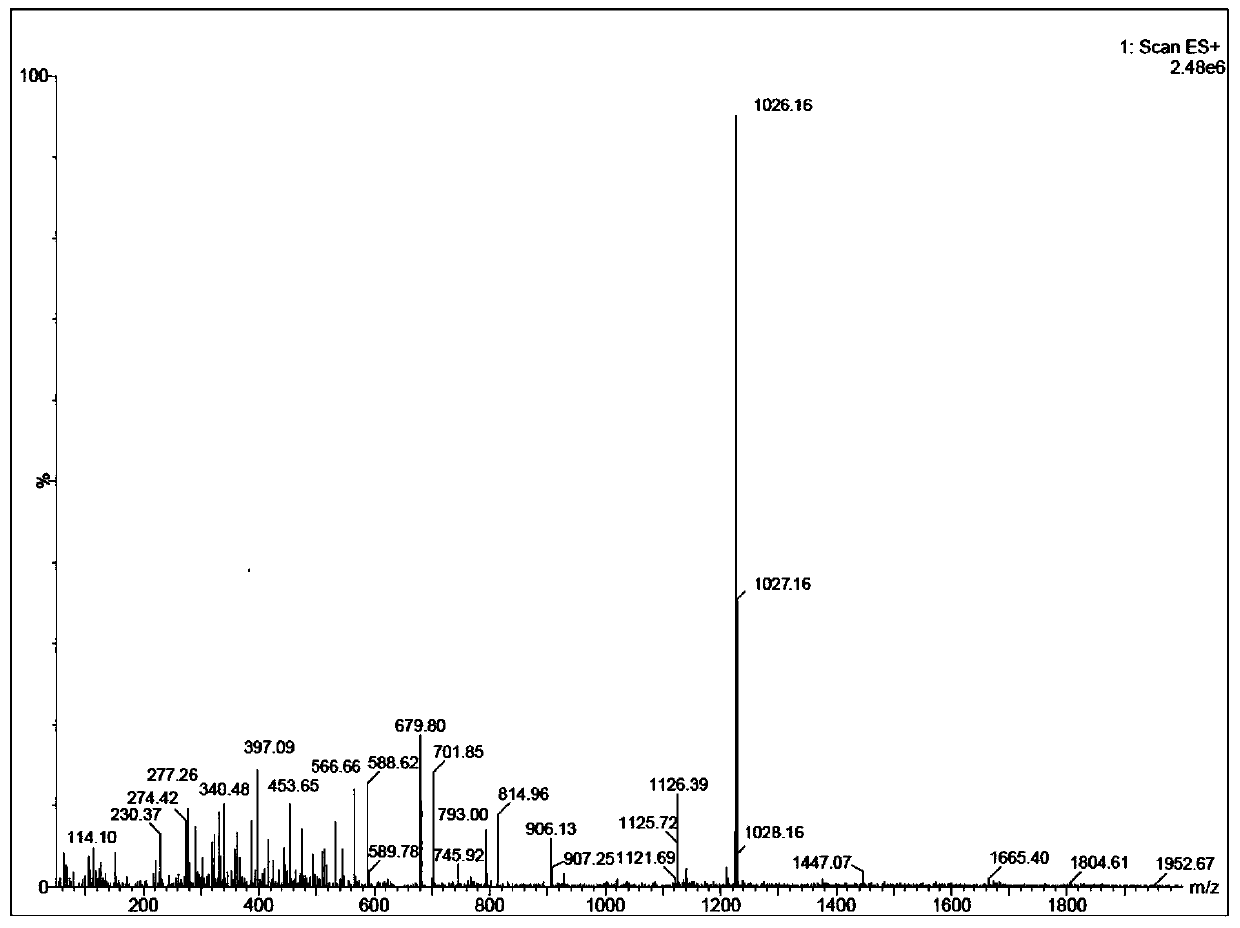
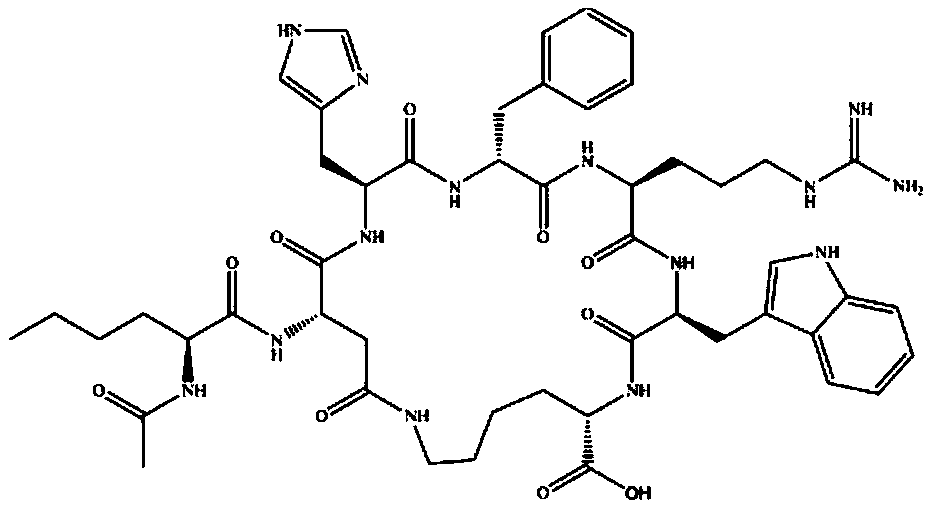
Solid-liquid phase synthesis method of Bremelanotide
A synthesis method and technology of bumenotide, which are applied in the field of solid-liquid phase synthesis of bumenotide, can solve the problems of large steric hindrance, difficult end-to-end connection, reduced synthesis yield, etc., and achieve low cost and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the synthesis of AC-Nle-OSu activated ester
[0054] Weigh 173.21g AC-Nle-OH (1.0mol), add 138.10g HOSu (1.2mol) into 2000ml DMF, add 247.59g DCC (1.2mol) under ice-water bath, react for 1 hour, heat up to room temperature for 3 hours, and react The liquid was filtered, the mother liquor was spin-dried, dissolved in DCM, filtered, recrystallized from ice ethanol three times, filtered, and dried by a solid oil pump to obtain 240.57g of AC-Nle-OSu activated ester with a yield of 89%.
Embodiment 2
[0055] Example 2: Synthesis of H-Asp-O-2-Phipr
[0056] Weigh 395.41g Fmoc-Asp(OAll)-OH (1.0mol), add it into 3000ml dichloromethane, add dropwise 561.16g 2,2,2-trichloroiminoacetic acid 2- Phenylpropan-2-yl ester (2.0 mol), react at room temperature for 16 hours. Under ice-cooling, 198.65 g of 2-phenylpropan-2-yl chloroformate (1.0 mol) dissolved in 2000 ml of dichloromethane was added dropwise, and reacted at room temperature for 16 hours. Concentrate the solution. Add 2000ml THF to dissolve, add 60g Pd(PPh 3 )4 (0.05mol) and 244mL PhSiH3 (2.0mol), react at room temperature for 2 hours. Concentrate the solution. 4000ml of dichloromethane and 1000ml of diisopropylethylamine. Reaction at room temperature for 0.5 hours. Concentrate the solution. Recrystallized from ice ethanol three times, filtered, and dried by pumping solid oil to obtain 201.02 g of H-Asp-O-2-Phipr, with a yield of 80%.
Embodiment 3
[0057] Example 3: Synthesis of AC-Nle-Asp-O-2-Phipr
[0058] Weigh 125.76g H-Asp-O-2-Phipr (0.5mol), 135.15g AC-Nle-OSu (0.5mol) and 79.50g Na 2 CO 3 (0.75mol) was added to a mixed solution of 500ml water and 500ml THF to dissolve, react overnight at room temperature, adjust the pH to 7 with 10% dilute hydrochloric acid, remove THF by rotary evaporation, and then adjust the pH to 3. A large white precipitate was obtained which was filtered. The obtained white precipitate was recrystallized with ice ethanol, the obtained solid was stirred and recrystallized in dioxane hydrochloride solution for 2 hours, and the obtained solid oil was pumped dry to 172.55g AC-Nle-Asp-O-2-Phipr, Yield 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com