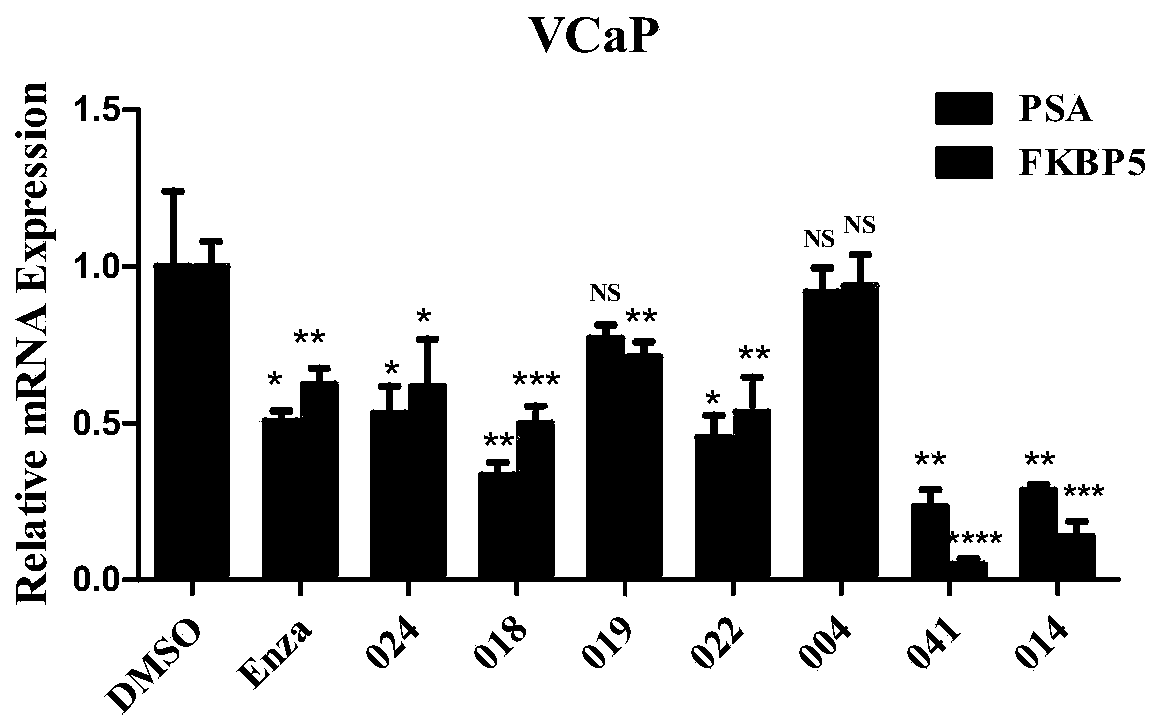
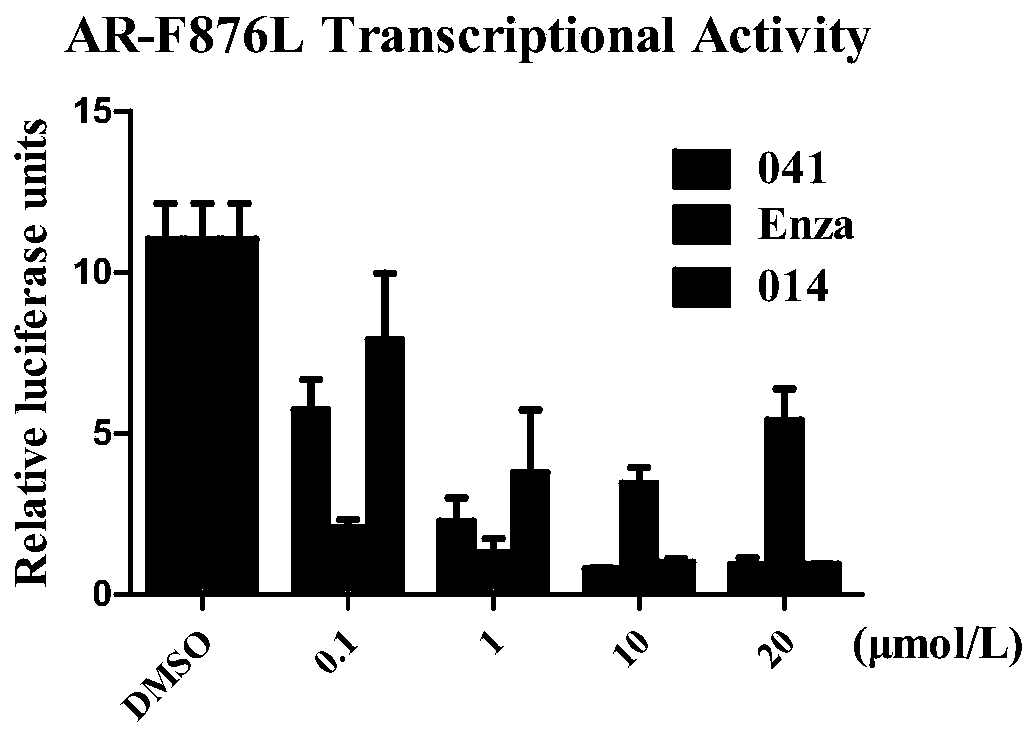
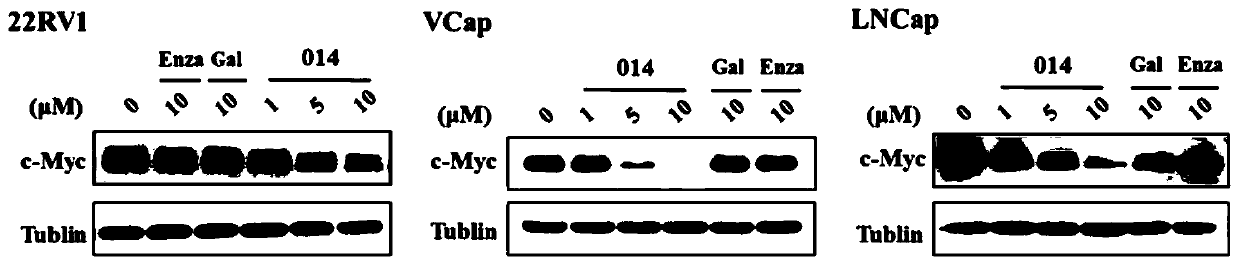
Dual inhibitors of AR and BET and use thereof
A technology of use and solvate, applied in the field of polycyclic compound and its double inhibitor of AR and BET
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1, the synthesis of compound among the present invention
[0076] The structure of each compound of the present invention in table 1
[0077]
[0078]
[0079]
[0080] (1) Synthesis of compound 001-036:
[0081] The preparation method of compound 001-036 is the same as the compound SKLB-C4558, SKLB-C4570, SKLB-C4561, SKLB-C4582, SKLB-C4567, SKLB-C4583, SKLB-C4579, SKLB-C4590, SKLB-C4596, SKLB-C4604 in CN107814785A , SKLB-C4613, SKLB-C4612, SKLB-C4614, SKLB-C4615, SKLB-C2602, SKLB-C4573, SKLB-C4578, SKLB-C4577, SKLB-C4574, SKLB-C4575, SKLB-C4576, SKLB-C4608, SKLB -C4609, SKLB-C4569, SKLB-C4572, SKLB-C2603, SKLB-C2601, SKLB-C4568, SKLB-C2604, SKLB-C4599, SKLB-C4602, SKLB-C4601, SKLB-C4565, SKLB-C4562, SKLB-C4563 , The preparation method of SKLB-C4560.
[0082] (2) Synthesis of Compound 037:
[0083] a. Deuterated ethanol-d6 (500mg, 10.8mmol) was dissolved in THF (2.7ml) and cooled to 0°C, NaOH (1.3g, 2.4ml) was dissolved in water and added after p...
Embodiment 7
[0176] Example 7 Androgen Receptor Binding Affinity Experiment
[0177] 1. Materials and instruments
[0178]LNCaP cell lysate, 3H-R1881 (PerkinElmer, Cat: NET590250UC, Lot: 2133648), glycerol (Sigma, Cat: G9012, Lot: BCBG6624V), 0.5M EDTA (Invitrogen-15575-038), DTT (Sigma, Cat: 43815, Lot: BCBD7009V), dextran (Sigma, Cat: D1662, Lot: SLBK5258V), activated carbon (Sigma, Cat: 05105, Lot: BCBF9839V), Tris base (Sigma, Cat: T1503-1KG), Protease inhibitor (PerkinElmer, Cat: 6013329, Lot: 77-16371), Scint-tube, 6ml (PerkinElmer, Cat: 6000192), 96-well plate (Agilent, Cat# 5042-1385), TopSeal sealing film (Perkin Elmer , Cat#6050185), Tri-Carb liquid scintillation analyzer (PerkinElmer, 2910TR)
[0179] 2. Method
[0180] The compound to be tested (the compound prepared in Example 1 of the present invention; the positive control Enzalutamide) was diluted with DMSO four times in a four-fold gradient, with a total of 8 concentration points, and 1 μl of each concentration was tran...
Embodiment 8
[0186] Example 8 BET protein binding affinity experiment
[0187] 1. Materials and instruments
[0188] BRD4(1,2)(Cat.No.31044);BRD2(1,2)(Cat.No.31024);BRD3(1,2)(Cat.No.31035);
[0189] BRD9 (Cat.No.B1048); CREBBP (Cat.No.31873); EP300 (Cat.No.31801);
[0190] SMARCA2 (Biogenie); FALZ (Cat.No.31447); TAF1 (D2, Biogenie).(+)-JQ1 (BPS, Cat.No.27402); EnSpire instrument (PerkinElmer, USA).
[0191] 2. Method
[0192] The inhibitory effect of the compound (the compound prepared in Example 1 of the present invention; positive control enzalutamide) on bromodomain-containing proteins BRD4 and BRD9 was evaluated by homogeneous time-resolved fluorescence (HTRF) technique. The test compound was serially diluted to 10 concentrations, with (+)-JQ1 (BPS, Cat. No. 27402) as the reference compound (Ref), and the final concentration of DMSO was 0.1%. Transfer the compound or DMSO to a 384-well assay plate, then add 2x Protein and Peptide Mix and 2x Detection Mix sequentially. After incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com