Method for removing pollutants by synergistically catalyzing persulfate with molybdenum disulfide and ferric ions
A persulfate and molybdenum disulfide technology, which is applied in the field of water pollution treatment, can solve the problems of easy precipitation, difficult circulation, and reduce the use of persulfate and ferric iron, so as to achieve low usage, low consumption, and iron reduction. The effect of sludge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
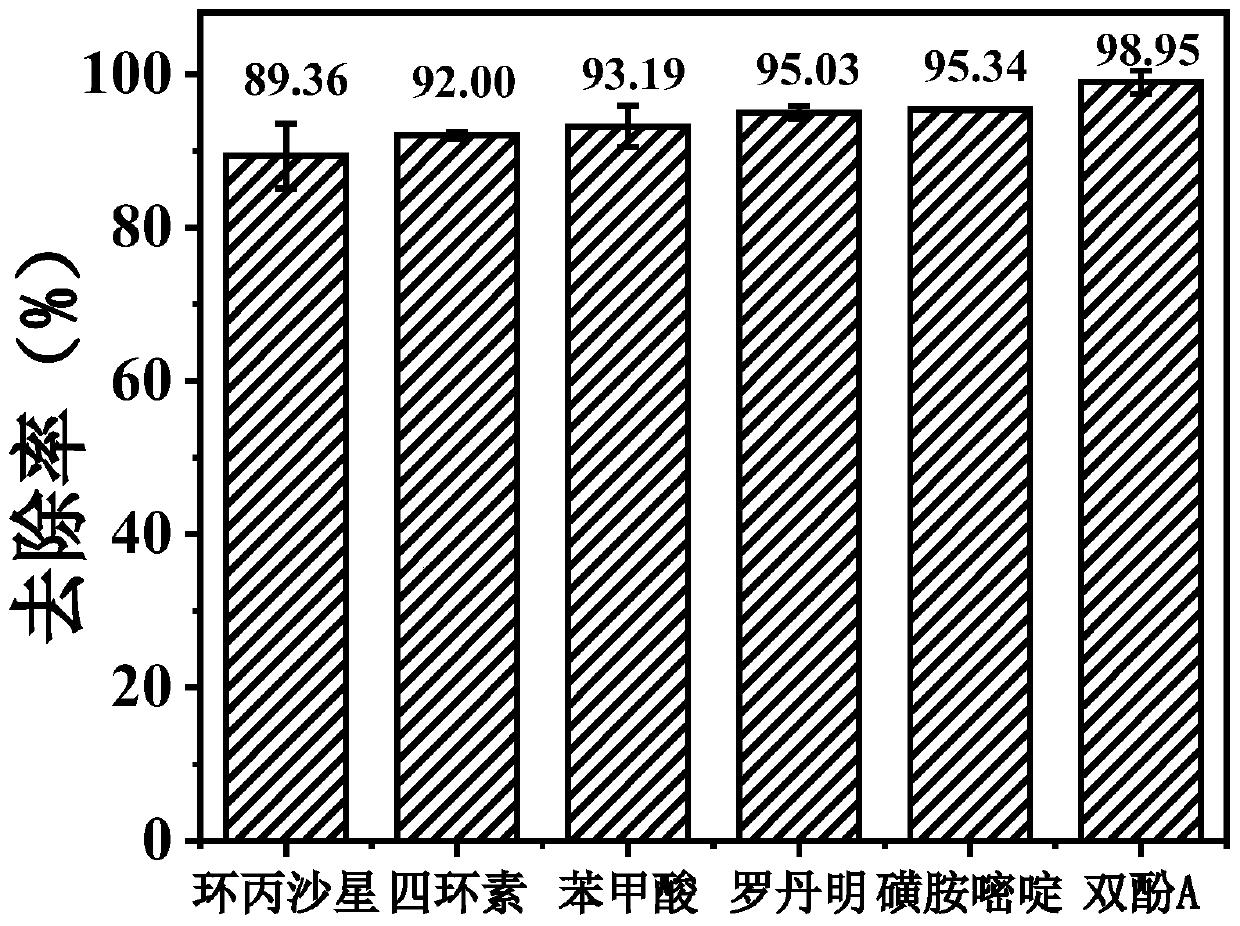
[0037] In this embodiment, at first molybdenum disulfide and Fe(III) ions synergistically catalyze and activate persulfate to degrade the degradation effect of each micro-pollutant in water, specifically:
[0038] Add 0.03g of molybdenum disulfide to a conical flask filled with 88ml of ultrapure water, mix with room temperature ultrasonic (power 300W) for 1min, add 10mL, 100mg / L rhodamine B aqueous solution, 1mM 10mM ferric chloride aqueous solution 1mL, and use 0.1 M sulfuric acid aqueous solution to adjust pH=3.0, stir evenly, then add 1mL of 150mM PMS aqueous solution to form a reaction system of 100ml, stir and react at room temperature, take 1ml of water sample before adding PMS as "zero point" (absorbance value is recorded as A0) With the water sample after reacting for 60min (the absorbance value is recorded as A1), the absorbance value is measured at 558nm by ultraviolet spectrophotometry, and the removal rate is calculated according to A1 / A0.
[0039] Under the same c...
Embodiment 2
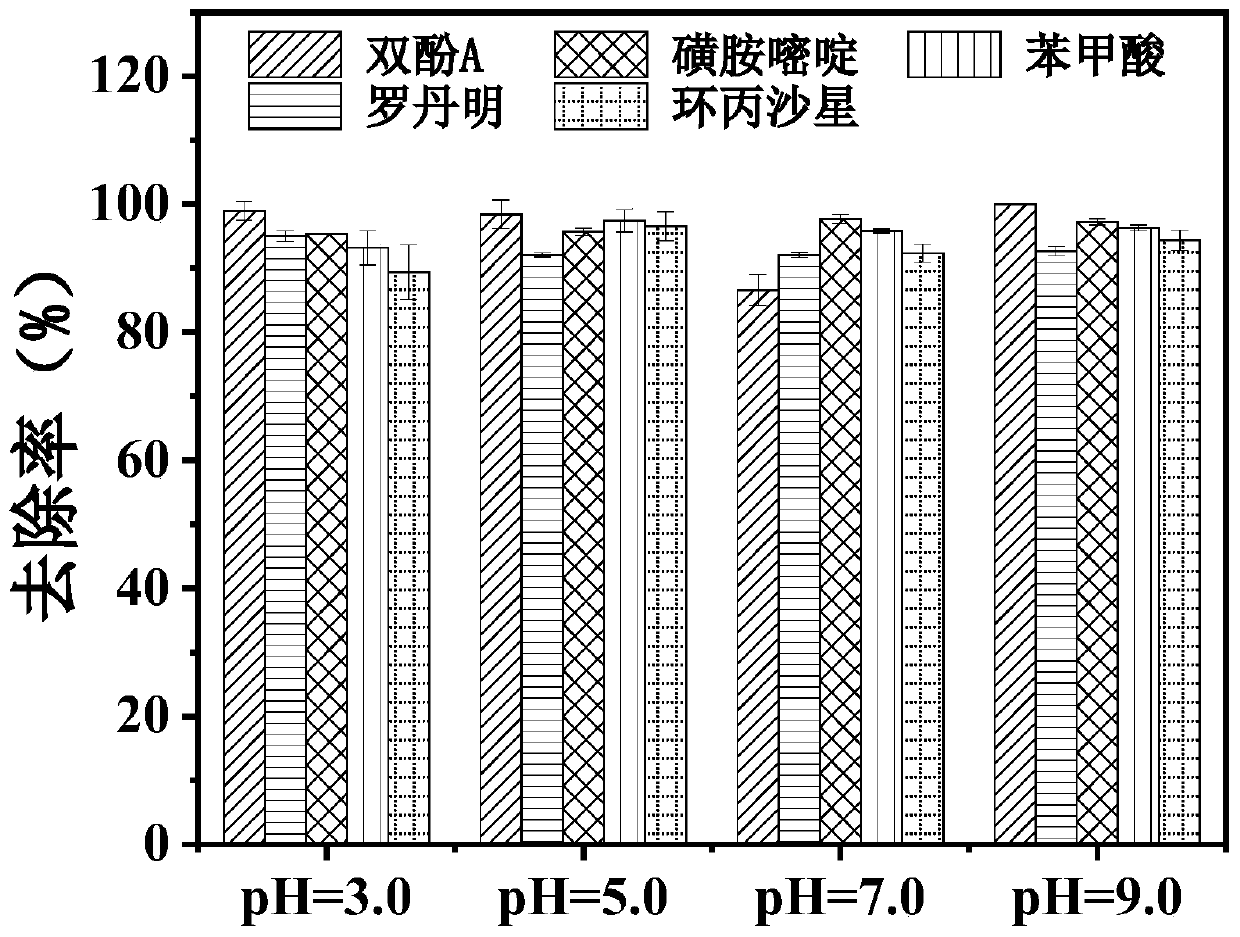
[0044] In this example, under different pH conditions, molybdenum disulfide and Fe(III) ions synergistically catalyze and activate persulfate to degrade the degradation effects of various micropollutants in water, specifically:
[0045] Add 0.03g of molybdenum disulfide to a conical flask filled with 88ml of ultrapure water, mix with room temperature ultrasonic (power 300W) for 1min, add 10mL, 100mg / L rhodamine B aqueous solution, 1mM 10mM ferric chloride aqueous solution 1mL, and use 0.1 M sulfuric acid aqueous solution or 0.1M potassium hydroxide aqueous solution to adjust pH=3.0~9.0 (3.0, 5.0, 7.0, 9.0), stir evenly, then add 1mL of 150mM PMS aqueous solution to form 100ml of reaction system, stir and react at room temperature, take 1ml of The water sample before adding PMS is "zero point" and the water sample after 60min, and its removal rate is calculated with the method of Example 1.
[0046] Under the same conditions, the rhodamine B aqueous solution was replaced with 5...
Embodiment 3
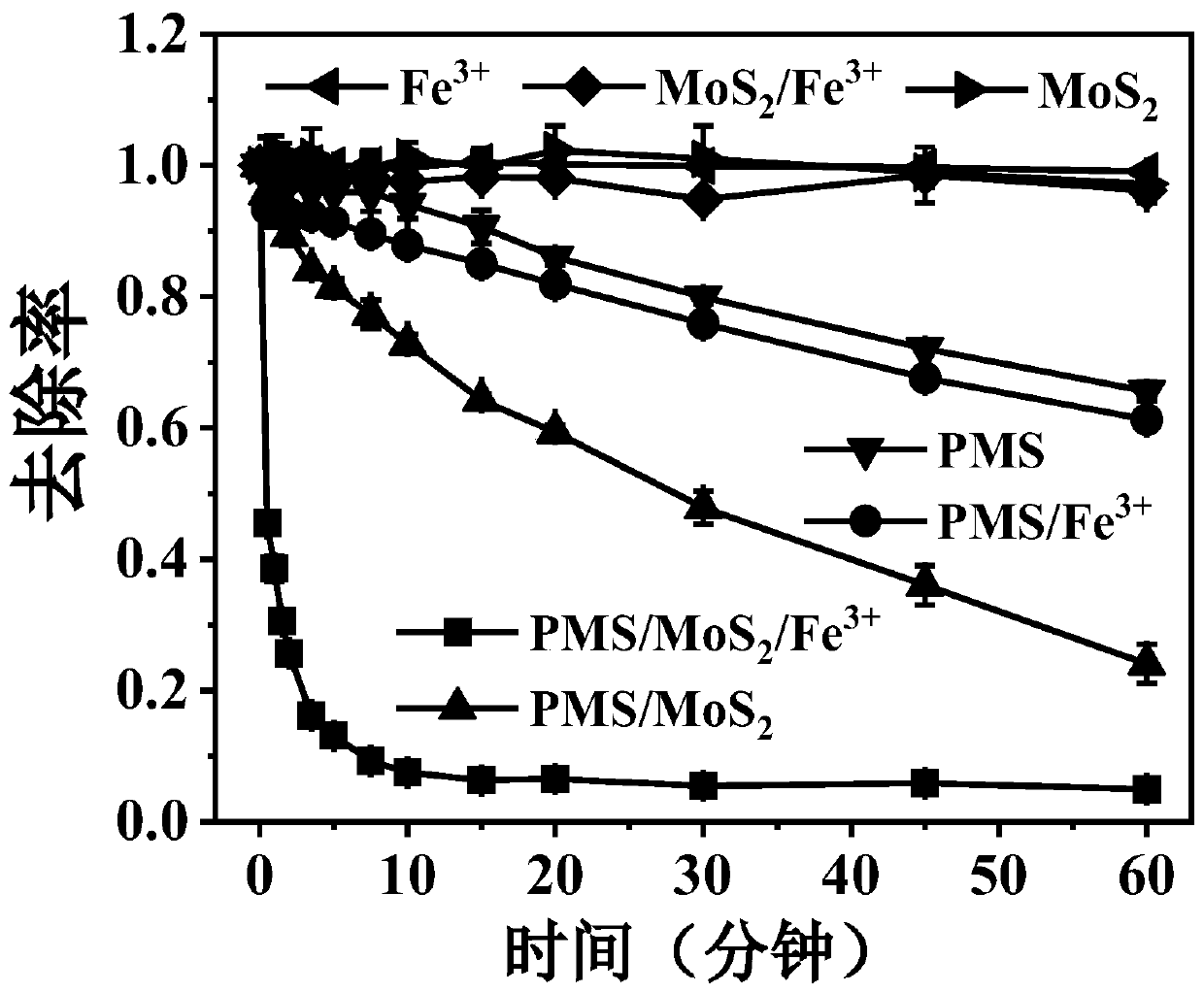
[0048] This example investigates the degradation effect of free radicals generated by catalytic activation of different control tests based on persulfate, molybdenum disulfide, and ferric iron to degrade pollutants in water.
[0049] Weigh Rhodamine B and dissolve it in water to prepare a rhodamine B aqueous solution with a concentration of 100mg / L for subsequent use; measure PMS and dissolve it in water to prepare a 150mM PMS aqueous solution for subsequent use; weigh ferric chloride hexahydrate solid and dissolve it in water Prepare a ferric chloride aqueous solution with a concentration of 10mM for later use.
[0050] The specific operation steps are as follows:
[0051] (1) Add 0.03g of molybdenum disulfide to a conical flask containing 90mL of ultrapure water, mix with room temperature ultrasonic (power 300W) for 1min, add 10mL of 100mg / L rhodamine B aqueous solution, and adjust the pH with 0.1M sulfuric acid aqueous solution= 3.0, stir evenly, carry out the degradation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com