Preparation method of denitration catalyst for glass kiln flue gas
A denitrification catalyst and glass kiln technology, applied in the field of flue gas denitrification treatment, can solve the problems of catalyst denitrification activity poisoning, reduce catalyst service life, etc., achieve the effect of improving low temperature denitrification activity, enhancing redox performance, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
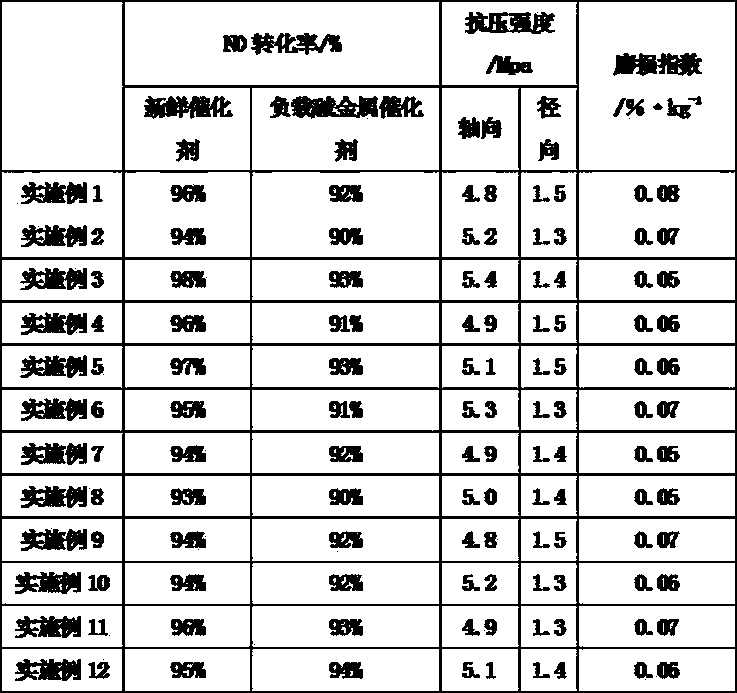
[0020] (1) In the reaction kettle, add a certain amount of 4.05kg oxalic acid and 270kg deionized water and heat to 90°C, add 0.64kg ammonium metavanadate, 7.36kg ammonium heptamolybdate, 3kg cerous nitrate and 0.5kg cobalt nitrate , stirred for a certain period of time to achieve full dissolution, and then 90kg TiO 2 10kg of expanded vermiculite was added to the mixed solution, and the temperature was raised to 180°C for 8 hours. The slurry-like material after the hydrothermal reaction was dried at 105° C. for 8 hours, and ground to obtain catalyst precursor powder.
[0021] (2) Weigh 100kg of catalyst precursor powder prepared in the above step (1), 1.2kg of carboxymethyl cellulose, 0.5kg of polyethylene oxide, 7kg of chopped basalt fiber, 3kg of ammonia water, 8kg of pulp cotton, and 0.8kg of stearic acid , adding 30kg of deionized water, after mud refining, vacuuming, aging under constant temperature and humidity conditions for 24 hours, forming, drying at 60°C for 48-72 ...
Embodiment 2
[0023] (1) In the reaction kettle, add a certain amount of 4.05kg oxalic acid and 270kg deionized water and heat to 100°C, add 0.64kg ammonium metavanadate, 7.36kg ammonium heptamolybdate, 3kg cerous nitrate and 0.5kg cobalt nitrate , stirred for a certain period of time to achieve full dissolution, and then 90kgTiO 2 10kg of expanded vermiculite was added to the mixed solution, and the temperature was raised to 200°C for 8 hours. The slurry-like material after the hydrothermal reaction was dried at 115° C. for 8 hours, and ground to obtain catalyst precursor powder.
[0024] (2) Weigh 100kg of catalyst precursor powder prepared in the above step (1), 1.2kg of carboxymethyl cellulose, 0.5kg of polyethylene oxide, 7kg of chopped basalt fiber, 3kg of ammonia water, 8kg of pulp cotton, and 0.8kg of stearic acid , adding 30kg of deionized water, after mud refining, vacuuming, aging under constant temperature and humidity conditions for 24 hours, forming, drying at 60°C for 48-72 ...
Embodiment 3
[0026] (1) In the reaction kettle, add a certain amount of 4.05kg oxalic acid and 270kg deionized water and heat to 120°C, add 0.64kg ammonium metavanadate, 7.36kg ammonium heptamolybdate, 3kg cerous nitrate and 0.5kg cobalt nitrate , stirred for a certain period of time to achieve full dissolution, and then 90kgTiO 2 10kg of expanded vermiculite was added to the mixed solution, and the temperature was raised to 220°C for 8 hours. The slurry-like material after the hydrothermal reaction was dried at 120° C. for 8 hours, and ground to obtain catalyst precursor powder.
[0027] (2) Weigh 100kg of catalyst precursor powder prepared in the above step (1), 1.2kg of carboxymethyl cellulose, 0.5kg of polyethylene oxide, 7kg of chopped basalt fiber, 3kg of ammonia water, 8kg of pulp cotton, and 0.8kg of stearic acid , adding 30kg of deionized water, after mud refining, vacuuming, aging under constant temperature and humidity conditions for 24 hours, forming, drying at 60°C for 48-72 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com