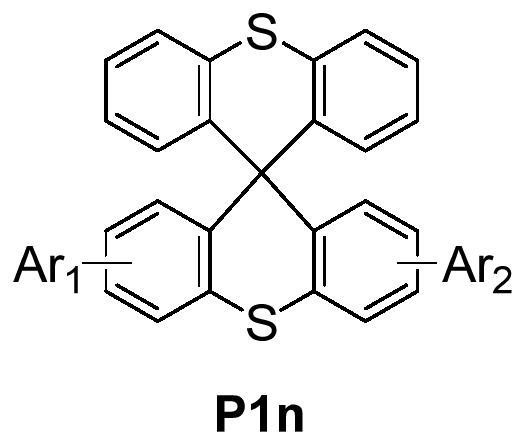
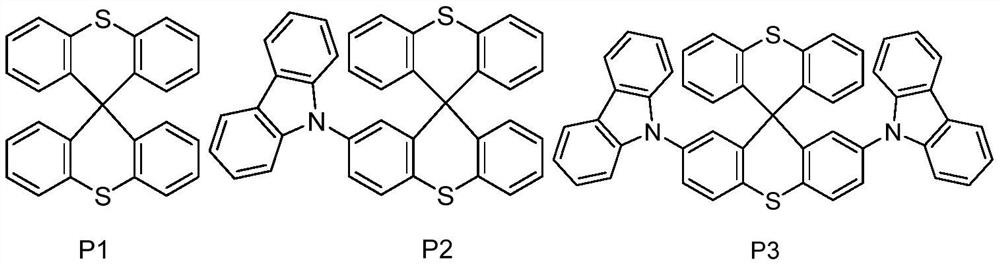
A small molecule hole transport material based on spiro-bisthioxanthene and its preparation method and application
A technology of hole transport material and thioxanthene, which is applied in semiconductor/solid-state device manufacturing, organic chemistry, electric solid-state devices, etc., can solve the problem that organic transport small molecule materials are rarely reported, and improve the carrier transport characteristics. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of P1:
[0039]
[0040] Preparation of P1: In a low-temperature reaction flask, add compound 1 (1-bromo-2phenylmercaptobenzene, preparation method reference: Schopfer, U.; Schlapbach, A.A general palladium-catalysed synthesis of aromatic and heteroaromatic thioethers. Tetrahedron 2001,57 , 3069-3073.) (2.65g, 100mmol), dissolved in 60ml THF, protected by nitrogen gas, sealed the device, cooled to -78°C with liquid nitrogen, added dropwise butyllithium (1.6mol / L in hexanes , 70mL, 110mmol), keep warm for 40min, then add 2.2g of thioxanthone in THF (50mL) at one time, and react overnight at room temperature. After the reaction is complete, THF is removed with a rotary evaporator, extracted with dichloromethane, and the product alcohol M3 is separated by a column. The product M3 was added with 20ml of acetic acid and 1.5mL of hydrochloric acid, under nitrogen protection, stirred overnight at 80°C, and separated by column to obtain 22.0g o...
Embodiment 2
[0041] Embodiment 2: the preparation of P3
[0042] Synthesis of intermediate product M4
[0043]
[0044] The synthesis method of M4 is as in P1. Add compound 1 (2.65g, 100mmol) into a low-temperature reaction flask, dissolve it with 60ml THF, and protect it with nitrogen gas. After sealing the device, add liquid nitrogen to cool to -78°C, and add dimethicone dropwise within 30min. base lithium (1.6mol / L inhexanes, 70mL, 110mmol), keep warm for 40min, then add 2.21g of 3,7-dibromothioxanthone in THF (50mL) at one time, and react overnight at room temperature. After the reaction was complete, THF was removed with a rotary evaporator, extracted with dichloromethane, and the product alcohol was separated by a column. The product alcohol was added to 20ml of acetic acid and 1.5mL of hydrochloric acid, protected by nitrogen, stirred at 80°C overnight, and separated by column to obtain 0.83g of white solid with a yield of 81%. 1H NMR: 7.22 - 7.18 (m, 4H), 7.04 (dd, J = 11.7, 4...
Embodiment 3
[0047] Embodiment 3: the synthesis of P2
[0048] Synthesis of intermediate product M5
[0049]
[0050] The synthesis method of M5 is as in P1. Add compound 1 (2.65g, 100mmol) into a low-temperature reaction flask, dissolve it with 60ml THF, and protect it with nitrogen gas. After sealing the device, add liquid nitrogen to cool to -78°C, and add D Base lithium (1.6mol / L inhexanes, 70mL, 110mmol), keep warm for 40min, then add 1.86g of 3-bromothioxanthone in THF (60mL) at one time, and react overnight at room temperature. After the reaction was complete, THF was removed with a rotary evaporator, extracted with dichloromethane, and the product alcohol was separated by a column. The product alcohol was added to 20ml of acetic acid and 1.5mL of hydrochloric acid, protected by nitrogen, stirred at 80°C overnight, and separated by column to obtain 0.65g of white solid with a yield of 81%. The molecular weight of the final product obtained by mass spectrometry: 457.98. Element...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com