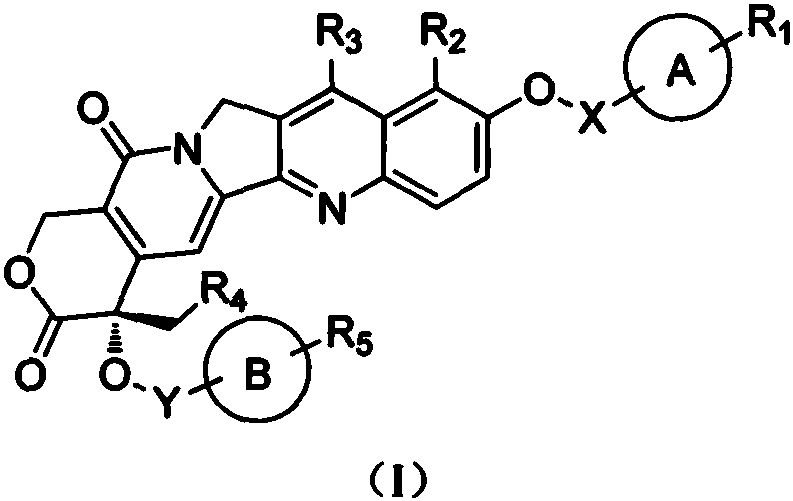
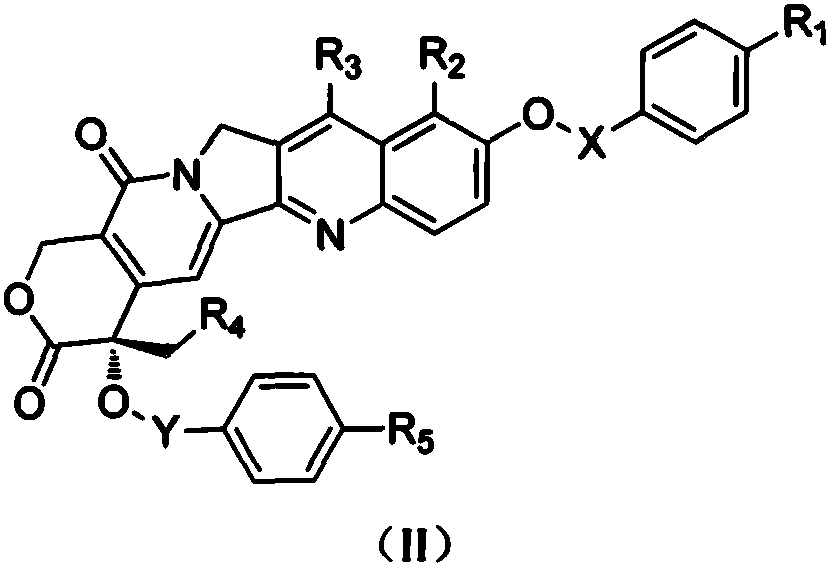
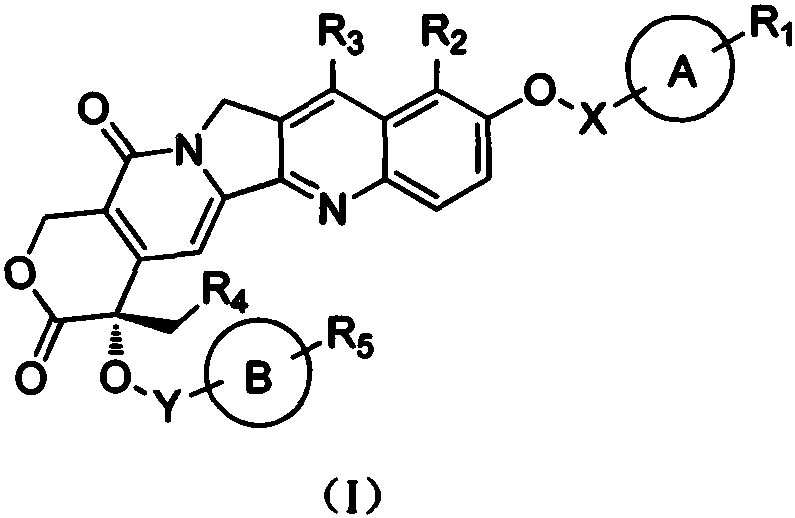
Camptothecin derivative and applications thereof
A technology of camptothecin and its derivatives is applied in the field of preparing anti-tumor drugs, which can solve the problems of lack of tumor tissue targeting, low activity, and large toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The camptothecin derivative of this example 4,11-diethyl-4-hydroxy-9-((4-(4,4,5,5-tetramethyl-1,3,2-oxopentaboron Alkyl-2-)phenyl)oxy)-1H-pyrano[3',4':6,7]indolazine[1,2-b]quinoline-3,14(4H,12H)-dione (No. SN-1) is synthesized through a one-step reaction, and the reaction formula is as follows.
[0063]
[0064] Dissolve 0.5 g (2.13 mmol) of 4-(hydroxymethyl) phenylboronic acid pinacol ester in 25 ml of dichloromethane, cool to 0°C, and protect with nitrogen. A solution of 0.2 ml (2.13 mmol) of phosphorus tribromide dissolved in 2 ml of dichloromethane was slowly added dropwise with a syringe, and the reaction was quenched by adding water for 0.5 h. After adding 25ml of dichloromethane, washed twice with 50ml of saturated sodium bicarbonate and 50ml of saturated sodium chloride solution respectively, retaining the organic phase, adding anhydrous sodium sulfate to dry, suction filtration and rotary evaporation to obtain 4-(bromomethyl) Pinacol phenylboronic acid est...
Embodiment 2
[0069]Tumor microenvironment (ROS)-mediated degradation of prodrugs: target compounds were dissolved in TE buffer (100uM), and 0.1mM H was added at 37°C 2 O 2 (or HO or HOCl) or buffer, add H by HPLC 2 O 2 Or the concentration of the target compound after 100s, 200s, 300s, 400s, 500s, 600s... of the buffer to determine whether the target compound can be degraded in the presence of ROS. The results showed that compound SN-1 could be slowly degraded under ROS conditions.
Embodiment 3
[0071] Cytotoxicity test: collect the log phase cells of HeLa cell line, adjust the concentration of cell suspension, add 100ul to each well, and plate the cells to adjust the density to 1000-10000 wells (the edge wells are filled with sterile PBS); 5% CO 2 , incubate at 37°C until the cell monolayer covers the bottom of the well (96-well flat bottom plate), add the drug with concentration gradient, set 5-7 concentration gradients, 5 duplicate wells, 100ul per well; 5% CO 2 , incubated at 37°C for 24h, and observed under an inverted microscope; 20ul MTT solution (5mg / ml, ie 0.5% MTT) was added to each well, and the culture was continued for 4h. If the drug can react with MTT, the culture medium can be discarded after centrifugation. Carefully rinse with PBS for 2-3 times, and then add the MTT-containing culture medium. The culture was terminated, and the culture medium in the well was carefully aspirated; 150 ul of dimethyl sulfoxide was added to each well, and it was shaken a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com