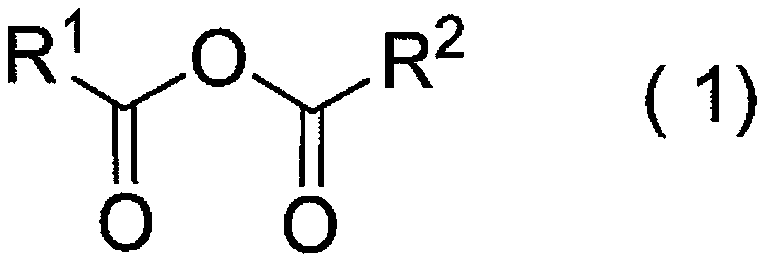
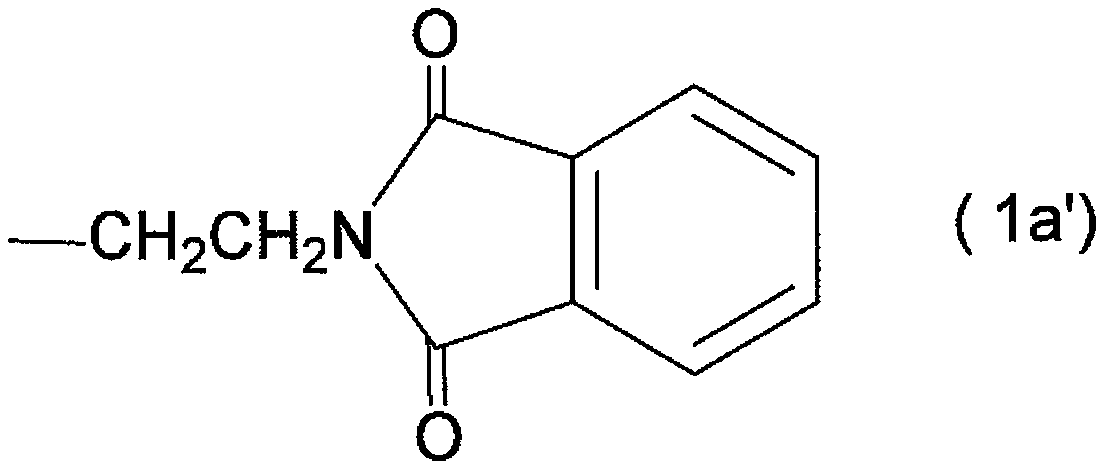
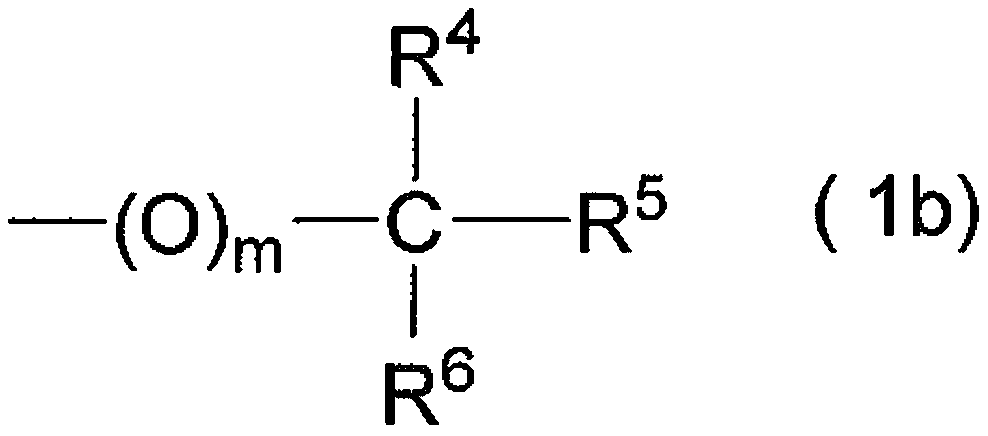
Protected l-carnosine derivative, l-carnosine, and method for producing crystalline l-carnosine zinc complex
A manufacturing method and protected technology, applied in the field of L-carnosine derivatives, can solve problems such as unstable yield and difficult purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0515] Example 1
[0516] The acid anhydride (II) represented by the formula (1") is produced according to the following reaction formula.
[0517]
[0518] Triphosgene (0.53 g, 1.79 mmol) in tetrahydrofuran (THF; 5 mL) was cooled below 7°C. In the manner that the temperature of the reaction solution is maintained below 10° C., the N-protected- A mixed liquid of β-alanine derivative; 1.00 g, 4.48 mmol), pyridine (organic base; 0.35 g, 4.42 mmol) and THF (5 mL). Then, the temperature of the reaction liquid was maintained at 10° C. or lower, and after stirring for 1 hour, the obtained reaction liquid was filtered under reduced pressure.
[0519] The resulting filtrate was mixed with an aqueous solution containing sodium bicarbonate at pH 8.0. The resulting liquid was extracted with ethyl acetate (10 mL). Then, the resulting ethyl acetate solution was washed to neutrality (pH 7) with an aqueous solution containing sodium bicarbonate at pH 8.0. The washed ethyl acetate so...
Embodiment 2
[0524] Example 2
[0525] According to the following reaction formula, the acid anhydride (II) represented by the formula (1") is reacted with L-histidine to produce the protected L-carnosine derivative (II) represented by the formula (3p2).
[0526]
[0527] A tetrahydrofuran (THF; 5 mL) solution containing triphosgene (0.53 g, 1.79 mmol) was cooled to below 7°C. With the temperature of the reaction solution maintained below 10°C, add dropwise the N-protected-β compound containing N-benzyloxycarbonyl-β-alanine (N-protection-β shown in formula (4)) to the THF solution containing triphosgene through 10 minutes. -A mixture of alanine derivative; 1.00 g, 4.48 mmol), pyridine (organic base; 0.35 g, 4.42 mmol) and THF (5 mL). Then, the temperature of the reaction solution is maintained below 10° C., and after stirring for 1 hour, the obtained reaction solution is filtered under reduced pressure to prepare 3-N-benzyloxycarbonylaminopropionic anhydride ( Anhydride (II)) filtrat...
Embodiment 3
[0530] Example 3
[0531] An acid anhydride (I) represented by formula (1') is produced according to the following reaction formula.
[0532]
[0533] Under a nitrogen atmosphere, prepare a mixture containing N-benzyloxycarbonyl-β-alanine (N-protected-β-alanine derivative shown in formula (4); 1.00 g, 4.48 mmol), triethylamine (organic base; 0.45 g, 4.45 mmol) and acetonitrile (7 mL), the solution was cooled to below 7°C. To the cooled solution, pivaloyl chloride (halide represented by formula (5); 0.54 g, 4.48 mmol) and acetonitrile (3 mL) were added dropwise over 10 minutes so that the temperature in the reaction liquid was maintained at 10° C. or lower. The solution. Stirring was performed for 1 hour while maintaining the temperature (10° C. or less) of the reaction liquid after the dropwise addition. Some crystals were taken out from the obtained reaction liquid, and the product was confirmed. The analysis results are as follows.
[0534] IR (KBr) 3339, 2976, 2939...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com