Pregabalin sustained release composition and preparation method thereof
A slow-release composition and technology of pregabalin, applied in the field of pregabalin preparations, can solve problems such as drug waste, uneven absorption of pregabalin, and inability of drugs to be effectively absorbed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
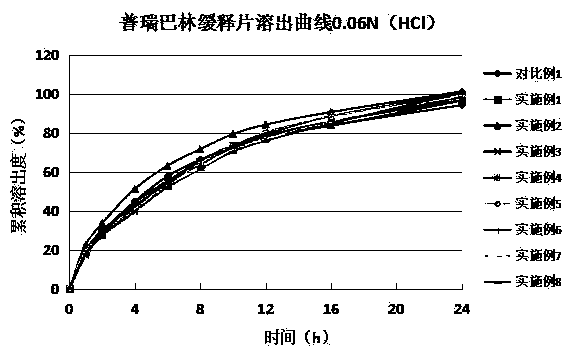
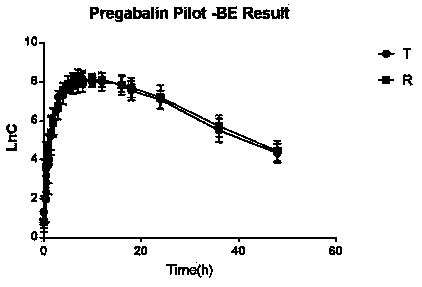
Image
Examples
Embodiment 1
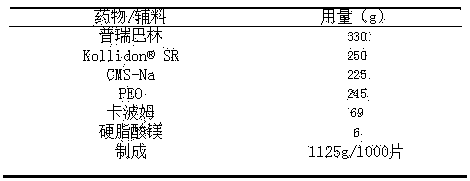
[0049] prescription:
[0050]
[0051] Preparation method: Weigh the prescribed amount of pregabalin, Kollidon® SR, CMS-Na and carbomer and pass through an 18-mesh sieve, then add PEO, mix with a mixer for 15 minutes, add magnesium stearate and mix for 5 minutes, and compress into tablets.
Embodiment 2
[0053] prescription:
[0054]
[0055] Preparation method: Weigh the prescribed amount of pregabalin, Kollidon® SR, carboxymethylcellulose calcium, and carbomer to pass through a 18-mesh sieve, then add PEO, mix with a mixer for 10 minutes, add half of the prescribed amount of magnesium stearate and mix for 5 minutes , dry granulation, pass through a 20-mesh sieve for granulation, then add half of the prescription amount of magnesium stearate, mix for 10 minutes, and compress into tablets.
Embodiment 3
[0057] prescription:
[0058]
[0059] Preparation method: Weigh the prescribed amount of pregabalin, Kollidon® SR, L-HPC and carbomer and pass through an 18-mesh sieve, then add PEO, mix in a mixer for 15 minutes, add magnesium stearate and mix for 5 minutes, and press into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap