Method for synthesizing linear and multifunctional polyester polyol by using double epoxy compound
A technology of diepoxy compound and polyester polyol, applied in the direction of adhesive type, polyurea/polyurethane adhesive, adhesive, etc., can solve the problem of unadjusted alkyd ratio and limited increase of polyester functionality problems, to avoid the problem of metal residues, increase the yield of polyester, and shorten the reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
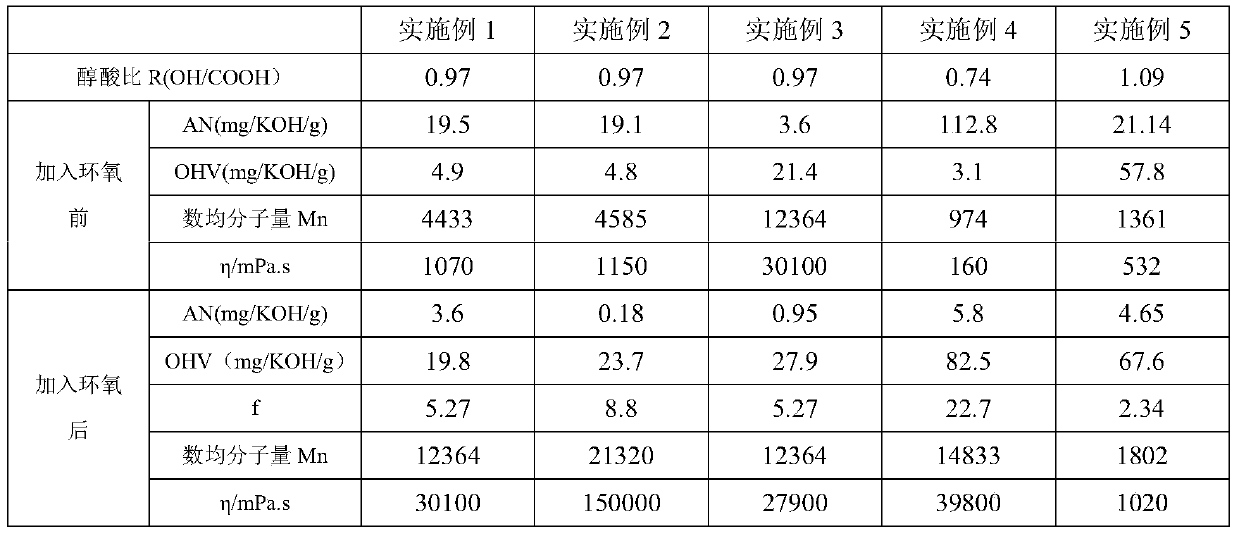
[0059] Example 1: Add 721.33g (6.10mol) of 1,6-hexanediol, 1278.67g (6.32mol) of sebacic acid, and 6g of antioxidant into a 2L four-necked flask, heat up and blow in nitrogen, and the system reaches Water began to flow out at 150°C, and the temperature was gradually raised to 230°C. After a period of heat preservation, 0.28 g of tetrabutyl titanate was added to continue the reaction. After a period of time, gradient vacuuming was started, and the temperature at the top of the reflux tower was maintained at a maximum of 90°C until the vacuum degree reached -0.095Mbar, keep vacuuming around 230°C, monitor the acid value and hydroxyl value, after about 13 hours, the measured AV=19.5mgKOH / g, release the vacuum, blow nitrogen, reduce the reaction temperature to 190-200°C, add bisphenol F-diglycidyl ether 74.0g, after 1h (AN=7.5mgKOH / g, OHV=12.52), add bisphenol F-diglycidyl ether 38.6g again, continue to react for 2h, measured AN=3.6mgKOH / g, The reaction was stopped, and the reacti...
Embodiment 2
[0060] Example 2: Add 721.33g (6.10mol) of 1,6-hexanediol, 1278.67g (6.32mol) of sebacic acid, and 6g of antioxidant into a 2L four-necked flask, heat up and blow in nitrogen, and the system reaches Water began to flow out at 150°C, and the temperature was gradually raised to 230°C. After a period of heat preservation, 0.28 g of tetrabutyl titanate was added to continue the reaction. After a period of time, gradient vacuuming was started, and the temperature at the top of the reflux tower was maintained at a maximum of 90°C until the vacuum degree reached -0.095Mbar, keep vacuuming at around 230°C, monitor the acid value and hydroxyl value, after about 13 hours, measure AV=19.1mgKOH / g, release the vacuum, blow nitrogen, reduce the reaction temperature to 190-200°C, add bisphenol F-diglycidyl ether 59.1g, after 1h (AN=11.7mgKOH / g, OHV=10.79), add bisphenol F-diglycidyl ether 45.2g again, continue to react for 2h (AN=4.6mgKOH / g, OHV= 16.98), add 9.7g of bisphenol F-diglycidyl et...
Embodiment 3
[0062]Embodiment 3: In order to make the acid value of the polyester in the embodiment 1 smaller, and no gel phenomenon occurs, a single epoxy compound is then added for end-capping. Take 1000.54g of the polyester of Example 1 into a 2L four-neck flask, add 15.08g of monoepoxy Cardura E10P, and react with nitrogen gas at 190-200°C for 2 hours to obtain a polyester product with an acid value of less than 2mgKOH / g. The test results are shown in Table 1 . Comparing this example with Example 1 and Example 2, it can be seen that the use of a single epoxy compound to end-cap the polyester polyol can effectively reduce the acid value (increase the reaction process), and maintain the functionality of the polyester polyol, The basic indicators of viscosity and molecular weight are equivalent to those after the diepoxy reaction, which effectively expands the application range of the polyester polyol. This example obtained the polyester product with acid value lower than 1 while obtaini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com