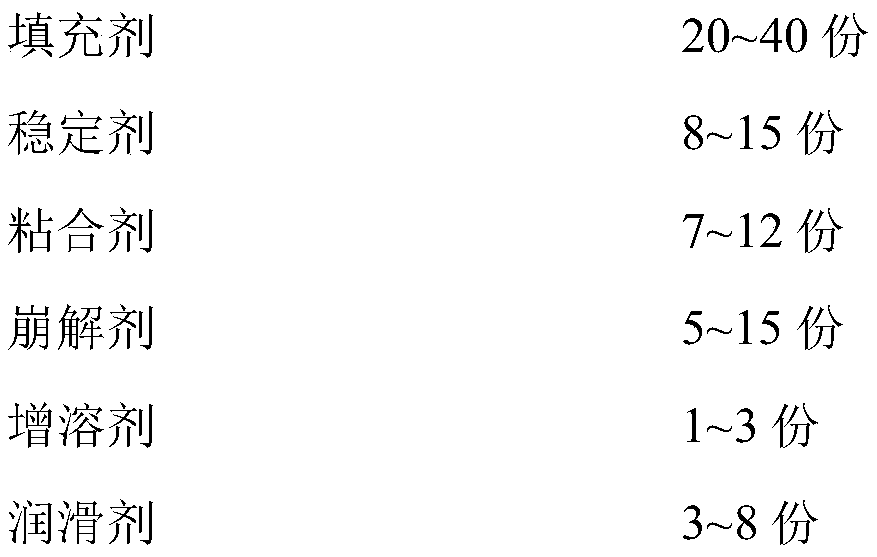Piribedil, levodopa and benserazide compound sustained-release three-layer tablet and preparation method thereof
A technology for piribedil and sustained-release tablets, which is applied in coatings, pill delivery, and pharmaceutical formulations. It can solve the problems of mutual influence between active ingredients, difficult long-term release of active ingredients, and increased impurity content, and achieve guaranteed Long-term release, guaranteed stability, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
[0055]
[0056] Its preparation method is:
[0057] (1) Mix the piribedil crude drug of the formula amount with the first layer of croscarmellose sodium, sucrose and calcium carbonate in a mixer, add the prepared adhesive containing polysorbate 80 After the wet granules are dried and granulated, colloidal silicon dioxide and magnesium stearate are added and mixed uniformly to obtain the first layer of granules after mixing;
[0058] (2) after mixing the second sheet of polyoxyethylene, sodium chloride, red iron oxide, and hypromellose in a mixer, dry granulation, adding magnesium stearate and mixing uniformly to obtain a second lamellar particles;
[0059] (3) The levodopa bulk drug, benserazide hydrochloride bulk drug and the third lamella lactose, microcrystalline cellulose, cross-linked polyvinylpyrrolidone, hypromellose, and colloidal silicon dioxide in the formula amount are mixed in Mix evenly in a mixer, dry granulate, add colloidal silicon dioxide and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com