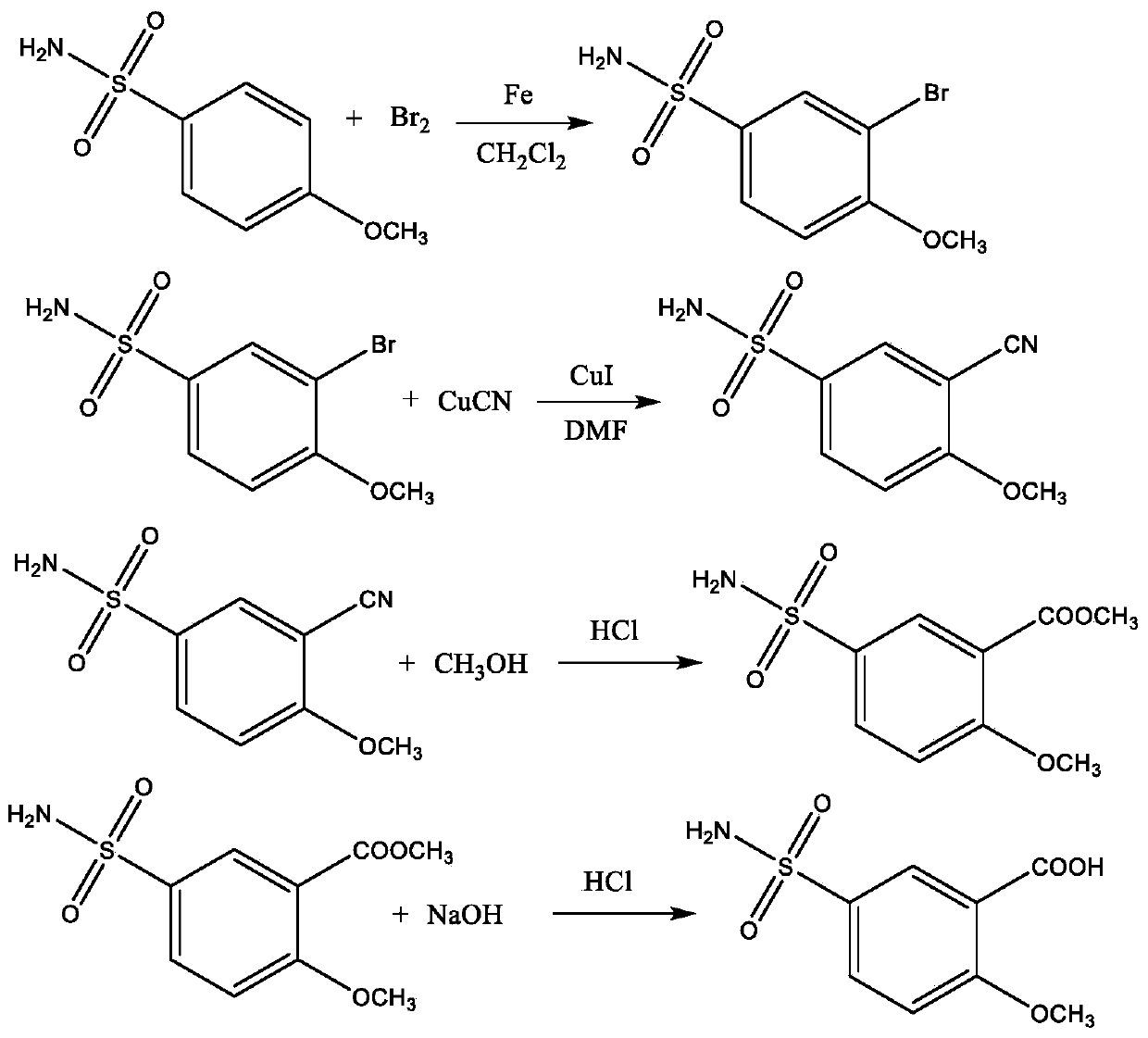
Preparation method of 2-methoxy-5-sulfamoylbenzoic acid
A technology of sulfonamidobenzoic acid and methoxybenzenesulfonamide, which is applied in the field of preparation of 2-methoxy-5-sulfonamidobenzoic acid, and can solve problems such as physical and environmental impacts and rare synthetic methods , to achieve the effect of improving selectivity, moderate price, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A preparation method of 2-methoxy-5-sulfonamidobenzoic acid, comprising the following steps:
[0024] (1) Add 187 g (1.0 mol) of 4-methoxybenzenesulfonamide, 10.29 g of iron powder, 88 g (1.1 mol) of bromine and 2618 g of methylene chloride into the reactor, and heat up to 60 °C under stirring , reacted at this temperature for 6 h, naturally cooled to room temperature after the reaction was completed, filtered, and the filtrate was evaporated to remove the solvent under normal pressure to obtain 223.44 g of the crude product of 3-bromo-4-methoxybenzenesulfonamide, with a yield of 84.0%;
[0025] (2) Add 266 g (1.0 mol) of 3-bromo-4-methoxybenzenesulfonamide prepared in step (1), 13.3 g of cuprous iodide, and 180 g of cuprous cyanide (2.0 mol) and 2128 g of N,N-dimethylformamide, heated to 120°C under stirring conditions, reacted at this temperature for 12 h, cooled naturally to room temperature after the reaction, filtered, evaporated the filtrate to remove the solvent ...
Embodiment 2
[0033] Other steps are the same as in Example 1, except that the preparation method of 3-bromo-4-methoxybenzenesulfonamide in step (1) is as follows:
[0034] 187 g (1.0 mol) of 4-methoxybenzenesulfonamide, 8.42 g of iron powder, 80 g (1.0 mol) of bromine and 748 g of dichloromethane were added to the reactor, and the temperature was raised to 40 °C under stirring conditions. Reaction at high temperature for 4 h, naturally cooled to room temperature after the reaction, filtered, and the filtrate was evaporated to remove the solvent under normal pressure to obtain 198.97 g of 3-bromo-4-methoxybenzenesulfonamide crude product, the yield was 74.8%, and it was detected by HPLC The content is 99.21%, and the product can be directly used in the next reaction without further purification.
Embodiment 3
[0036]Other steps are the same as in Example 1, except that the preparation method of 3-bromo-4-methoxybenzenesulfonamide in step (1) is as follows:
[0037] Add 187 g (1.0 mol) of 4-methoxybenzenesulfonamide, 9.35 g of iron powder, 84 g (1.05 mol) of bromine and 1683 g of dichloromethane into the reactor, and raise the temperature to 50 °C under stirring conditions. Reaction at high temperature for 5 h, naturally cooled to room temperature after the reaction was completed, filtered, and the filtrate was evaporated to remove the solvent under normal pressure to obtain 206.42 g of 3-bromo-4-methoxybenzenesulfonamide crude product, yield 77.6%, detected by HPLC The content is 99.13%, and the product can be directly used in the next reaction without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com