Synthesis method of alkali metal trifluoromethanesulfonate
A technology of trifluoromethanesulfonic acid base and synthesis method, which is applied in the preparation of sulfonate, the preparation of sulfonate, organic chemistry, etc., can solve the problem that the purity of lithium trifluoromethanesulfonate cannot reach the standard, the conductivity of the battery is affected, and the moisture cannot be Complete removal of problems such as avoiding the introduction of impurities, low cost and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
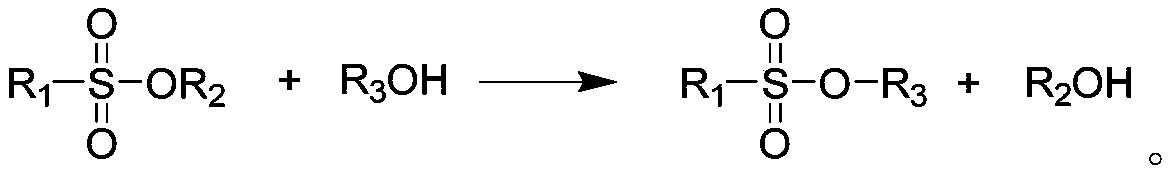
[0029] Add 111g (1.0mol) of anhydrous calcium chloride and 64g (2mol) of methanol into reactor I equipped with a thermometer, a distillation device, and mechanical stirring, evacuate the system to -0.095MPa, cool the system to -40°C, and Add 380 g (2.5 mol) of trifluoromethanesulfonyl fluoride. Stir mechanically, control the temperature of the system at 0°C, and the pressure at 0.1 MPa, and react for 2.5 hours. After the reaction, slowly open the outlet valve of the system to release excess trifluoromethanesulfonyl fluoride and collect it by cooling. After the pressure in the reaction vessel I was reduced to normal pressure, the temperature of the system was slowly raised for distillation, and the fractions at a temperature of 90-105°C were collected to obtain 304 g of methyl trifluoromethanesulfonate with a yield of 92.7%.
[0030] Take 164g (1.0mol) of methyl trifluoromethanesulfonate in reactor II equipped with a thermometer and mechanical stirring, and add 44g (1.0mol) of...
Embodiment 2
[0032] Add 111g (1.0mol) of calcium chloride and 96.6g (2.1mol) of ethanol into reactor I equipped with a thermometer, a distillation device, and mechanical stirring, evacuate the system to -0.095MPa, cool the system to -40°C, and Add 380 g (2.5 mol) of trifluoromethanesulfonyl fluoride. Stir mechanically, control the temperature of the system at 0°C, and the pressure at 0.05 MPa, and react for 3 hours. After the reaction, slowly open the outlet valve of the system to release excess trifluoromethanesulfonyl fluoride and collect it by cooling. After the pressure in the reaction vessel I was reduced to normal pressure, the temperature of the system was slowly raised for distillation, and the fractions at a temperature of 90-105°C were collected to obtain 316.8 g of ethyl trifluoromethanesulfonate with a yield of 88.9%.
[0033] Get 178g (1.0mol) of ethyl trifluoromethanesulfonate in reactor II equipped with a thermometer and mechanical stirring, add 40g (1.0mol) of sodium hydro...
Embodiment 3
[0035] Add 111g (1.0mol) of calcium chloride and 64g (2.0mol) of methanol into the reactor I equipped with a thermometer, a distillation device, and mechanical stirring, and evacuate the system to -0.095MPa. 245 g (2.5 mol) of sulfonyl fluoride. Stir mechanically, control the temperature of the system at 68°C, and react at a pressure of 0.1MPa for 2h. After the reaction, slowly open the outlet valve of the system to release excess methanesulfonyl fluoride and collect it under cooling. Slowly raise the system temperature and distill under reduced pressure, the pressure is -0.095MPa, and the fractions with a temperature of 90-95°C are collected to obtain 204g of methyl methanesulfonate, with a yield of 92.7%.
[0036] Take 110 g (1.0 mol) of methyl methanesulfonate in reactor II equipped with a thermometer and mechanical stirring, and add 44 g (1.0 mol) of lithium hydroxide monohydrate. Stir mechanically, control the temperature of the system at 50°C, and react at a pressure o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com