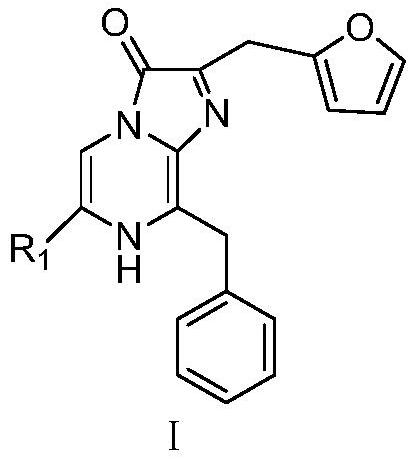
A kind of c-6 modified nanoluc type analogue and its preparation method and application
A kind of analogue and type technology, which is applied in the field of modification and preparation of NanoLuc type analogues at the C-6 position, can solve the problems of poor penetration into tissues and skin, poor substrate stability, short emission wavelength, etc., and achieve design and synthesis Effects of facilitation, improved stability, and low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
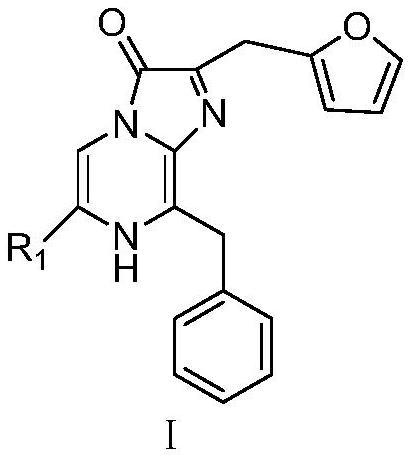
[0087] Example 1: 2-(furan-2-methyl)-6-(4-fluorophenyl)-8-imidazo[1,2-a]pyrazin-3-(7H)-one (A1) preparation.
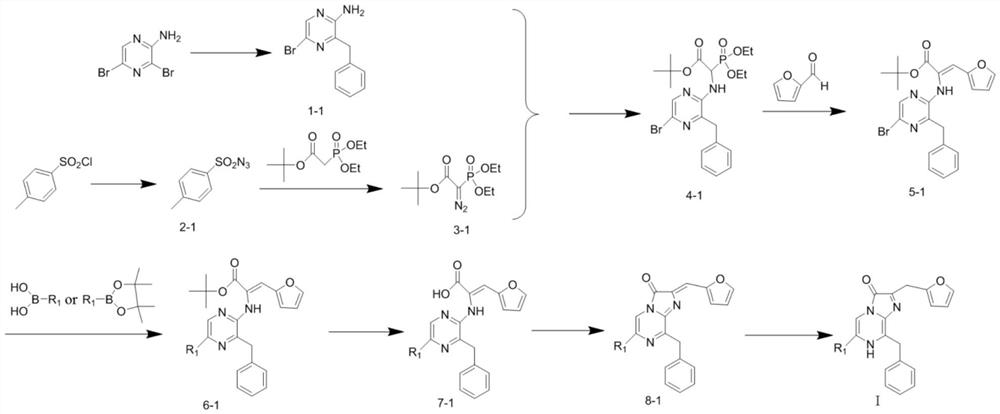
[0088] Preparation of intermediate 2-amino-5-bromo-3-phenylmethylpyrazine (intermediate 1-1):
[0089]Under nitrogen protection, mix activated zinc powder (5 g, 77 mmol) and elemental iodine (500 mg), add DMF, and stir at room temperature for 10 minutes until the color of iodine disappears. Then benzyl bromide (3.6ml, 25.6mmol) was added and refluxed in an oil bath at 85°C for 3h. After 3h, it was moved to room temperature, and 2-amino-3,5-dibromopyrazine (5 g, 20 mmol) and bistriphenylphosphine palladium dichloride (700 mg, 1 mmol) were added. Reaction at room temperature for 12h. The reaction solution was filtered with diatomaceous earth, ethyl acetate and saturated ammonium chloride solution were added to extract repeatedly three times, the ethyl acetate phase was dried with anhydrous sodium sulfate, filtered, and purified on 200-300 mesh silica gel to obtain in...
Embodiment 2
[0106] Example 2: 2-(furan-2-methyl)-6-(4-chlorophenyl)-8-benzylimidazo[1,2-a]pyrazin-3-(7H)-one (A2 ) preparation.
[0107] Preparation of intermediate 2-((3-benzyl-5-(4-chlorophenyl)pyrazin-2-yl)amino)-3-(furan-2-yl)acrylate tert-butyl ester:
[0108] Under nitrogen protection conditions, 2-((3-benzyl-5-bromopyrazin-2-yl)amino)-3-(furan-2-yl) tert-butyl acrylate (200mg, 0.438mmol), Pd( PPh 3 ) 4 (50.6mg, 0.0438mmol) was dissolved in 1,4-dioxane, then a solution of 4-chlorophenylboronic acid (136.7mg, 0.876mmol) in 1,4-dioxane was added, and Na 2 CO 3 (2M, 0.2ml), heated to reflux at 85°C for 4h. After the reaction was terminated, it was extracted three times by adding distilled water and ethyl acetate, dried over anhydrous sodium sulfate, filtered, and purified on 200-300 mesh silica gel to obtain 186.2 mg of a yellow solid with a yield of 87.3%.
[0109] Preparation of intermediate 2-((3-benzyl-5-(4-chlorophenyl)pyrazin-2-yl)amino)-3-(furan-2-yl)acrylic acid:
[0110...
Embodiment 3
[0115] Example 3: 2-(furan-2-methyl)-6-(thienyl-2)-8-benzylimidazo[1,2-a]pyrazin-3-(7H)-one (A5) preparation.
[0116] Preparation of intermediate 2-((3-benzyl-5-(thienyl-2)pyrazin-2-yl)amino)-3-(furan-2-yl)acrylate tert-butyl ester
[0117] Under nitrogen protection conditions, 2-((3-benzyl-5-bromopyrazin-2-yl)amino)-3-(furan-2-yl) tert-butyl acrylate (200mg, 0.438mmol), Pd( PPh 3 ) 4 (50.6mg, 0.0438mmol) was dissolved in 1,4-dioxane, then a solution of 2-thiopheneboronic acid (112.1mg, 0.876mmol) in 1,4-dioxane was added, and Na 2 CO 3 (2M, 0.2ml), heated to reflux at 85°C for 4h. After the reaction was terminated, distilled water and ethyl acetate were added to extract three times, dried over anhydrous sodium sulfate, filtered, and purified on 200-300 mesh silica gel to obtain 153.8 mg of a yellow solid with a yield of 72.1%.
[0118] Preparation of intermediate 2-((3-benzyl-5-(thienyl-2)pyrazin-2-yl)amino)-3-(furan-2-yl)acrylic acid:
[0119] 2-((3-Benzyl-5-(thienyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com