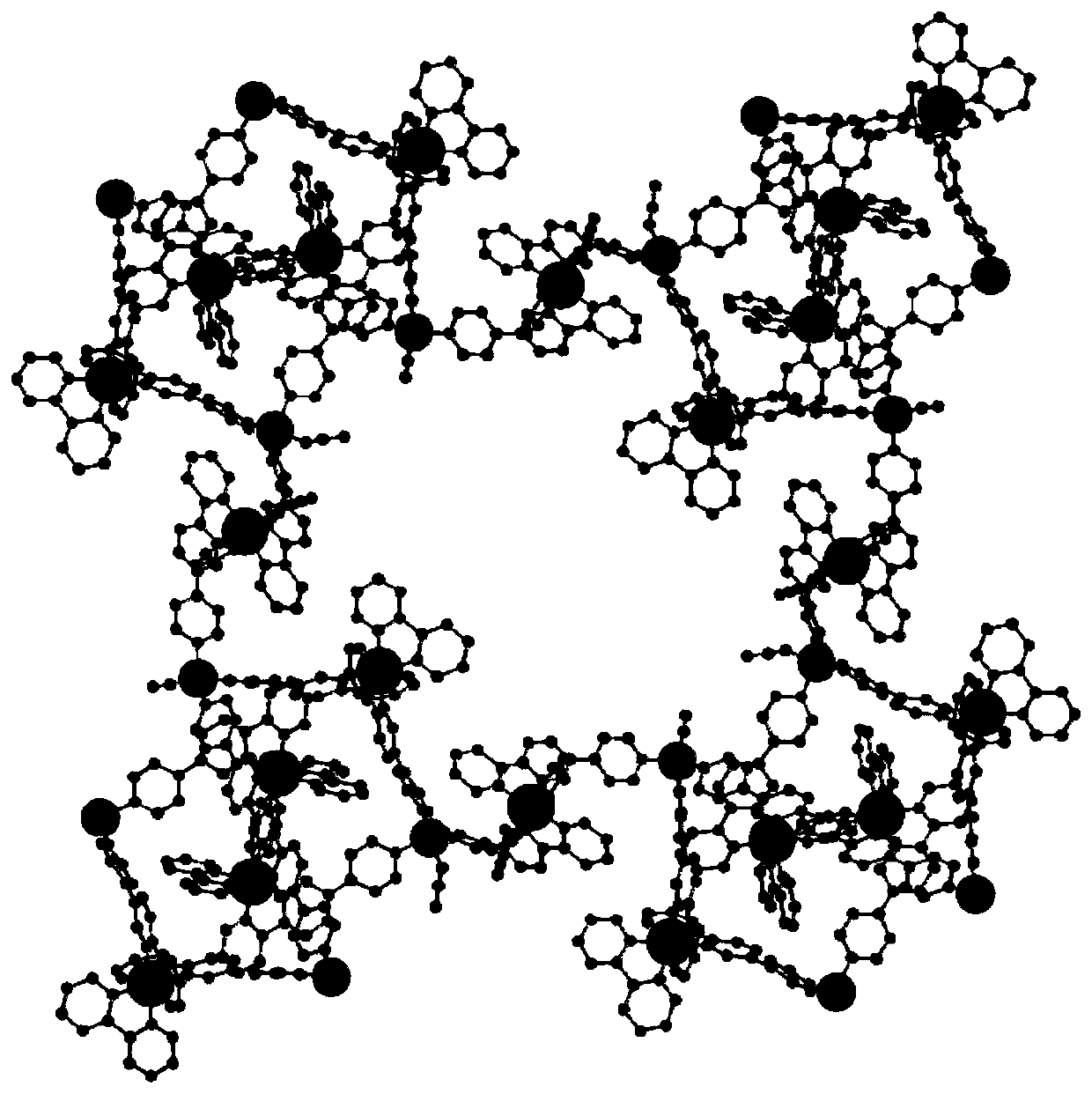
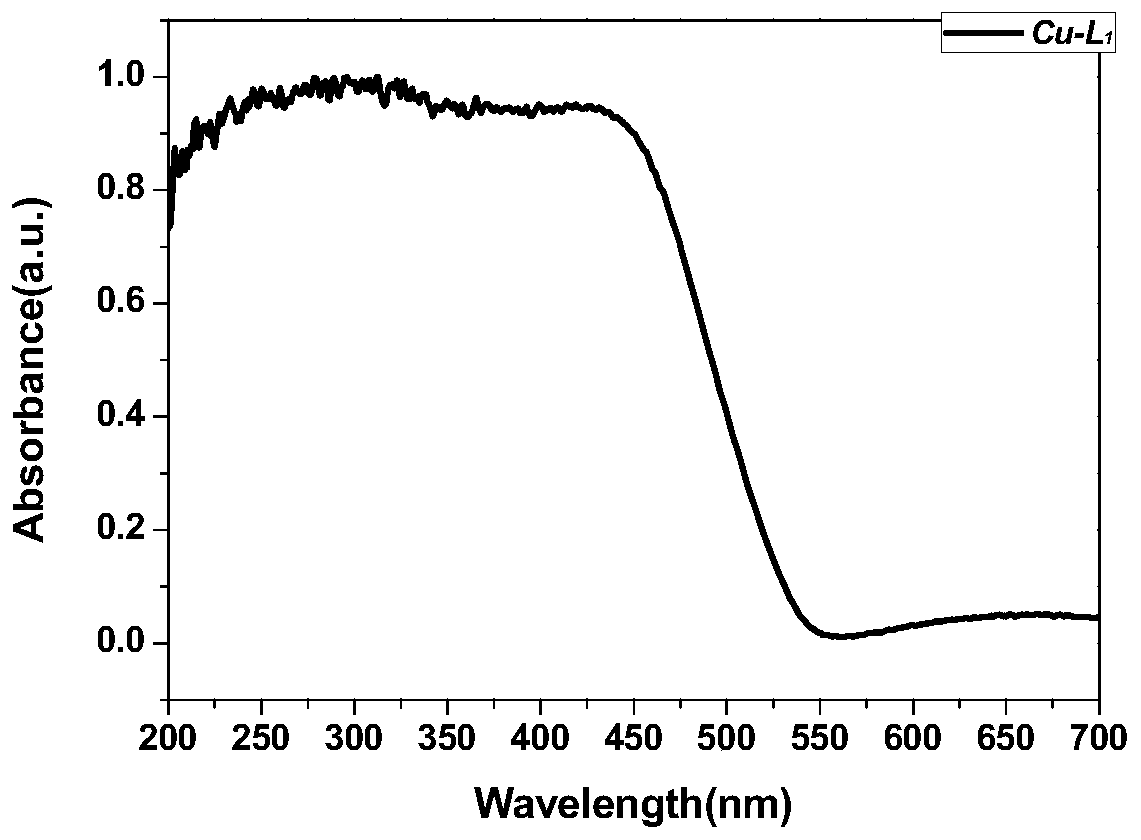
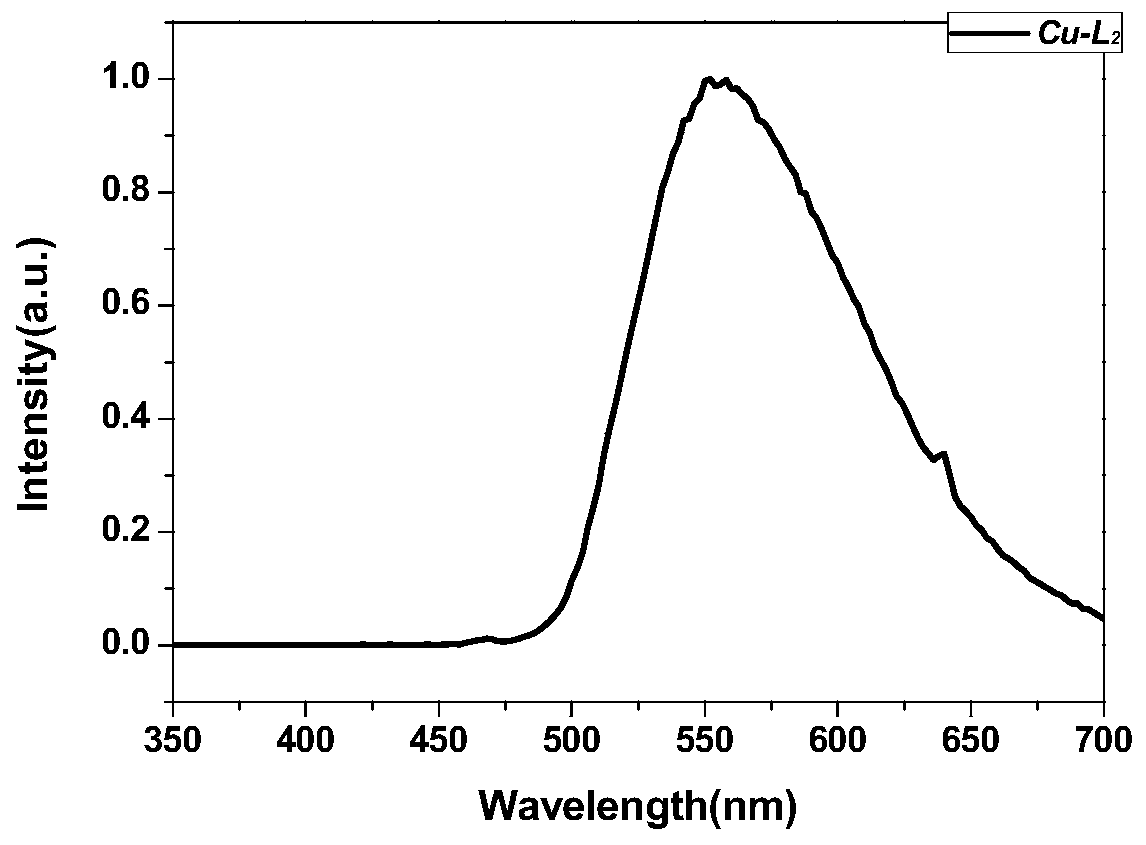
Preparation method and application of metal-organic framework structure compound with chiral pore channel structure
A technology of pore structure and organic framework, applied in the direction of indium organic compounds, platinum group organic compounds, organic compounds/hydrides/coordination complex catalysts, etc. Good recycling, high price and other issues, to achieve the effect of chiral cavity photocatalytic properties, excellent photocatalytic properties, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 4-Bromophenylboronic acid (2.4g, 12mmol), potassium carbonate (3.32g, 24mmol) and palladium acetate (36mg, 0.16mmol) were added to 40mL of a mixed solvent of ethanol and water with a volume ratio of 3:1, and then 2 -Bromopyridine (790uL, 8mmol), react at 80°C for 2h. Cooled to room temperature, extracted with ethyl acetate and saturated brine, dried over anhydrous sodium sulfate, filtered and rotary evaporated, and passed through a silica gel column with a mixed solution of petroleum ether and ethyl acetate at a volume ratio of 100:1 to obtain 1.6 g of a white powder. Yield 86%. Add iridium trichloride (762.7mg, 2.54mmol) and white powder (1.5g, 6.44mmol) to 30mL of a mixed solvent of ethylene glycol monomethyl ether and water at a volume ratio of 3:1, and at 120°C Stir under reflux for 24h, after the reaction is completed, suction filter and wash the filter cake obtained by suction filtration with a small amount of water, ethanol and ether, and vacuum dry the filter c...
Embodiment 2
[0044] The right-handed helical chiral metal-based ligand Ir(III) complex L 1 (24mg, 0.025mmol) and tetraacetonitrile copper tetrafluoroborate (15.7mg, 0.05mmol) were added to 10mL of acetonitrile solvent, stirred at room temperature for 4h, after filtration, the filtrate was left at room temperature for 2 weeks, and green Solid, the target compound Cu-L was obtained 1 20 mg, yield 40%.
Embodiment 3
[0046] The right-handed helical chiral metal-based ligand Ir(III) complex L 1 (24mg, 0.025mmol) and tetraacetonitrile copper hexafluorophosphate (18.6mg, 0.05mmol) were added to 10mL of acetonitrile solvent, stirred at room temperature for 4h, after filtration, the filtrate was left at room temperature for 2 weeks, and green Solid, the target compound Cu-L was obtained 1 19 mg, yield 38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com