Single-domain antibody combined with immunoglobulin, anti-avian influenza single-domain antibody, bifunctional antibody and application thereof
An immunoglobulin and single-domain antibody technology, which is applied to immunoglobulin-binding single-domain antibodies, anti-avian influenza single-domain antibodies, bifunctional antibodies and their application fields, and can solve the problems of short elimination half-life and small molecules.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Construction of Human Immunoglobulin (Ig) Specific Single Domain Antibody Gene Library
[0070] 1. Alpaca immunization procedure, monitoring of specific single domain antibody production
[0071] (1) Alpaca immunization procedure: multi-point subcutaneous injection of alpaca neck and back, human IgG and IgA antigens and Freund's complete adjuvant equal volumes were prepared into an emulsion, and the first immunization interval was 4 weeks; For 4 immunizations, an equal volume of human IgG and IgA antigens and Freund's incomplete adjuvant was prepared into an emulsion, and the interval between each immunization was 3 weeks.
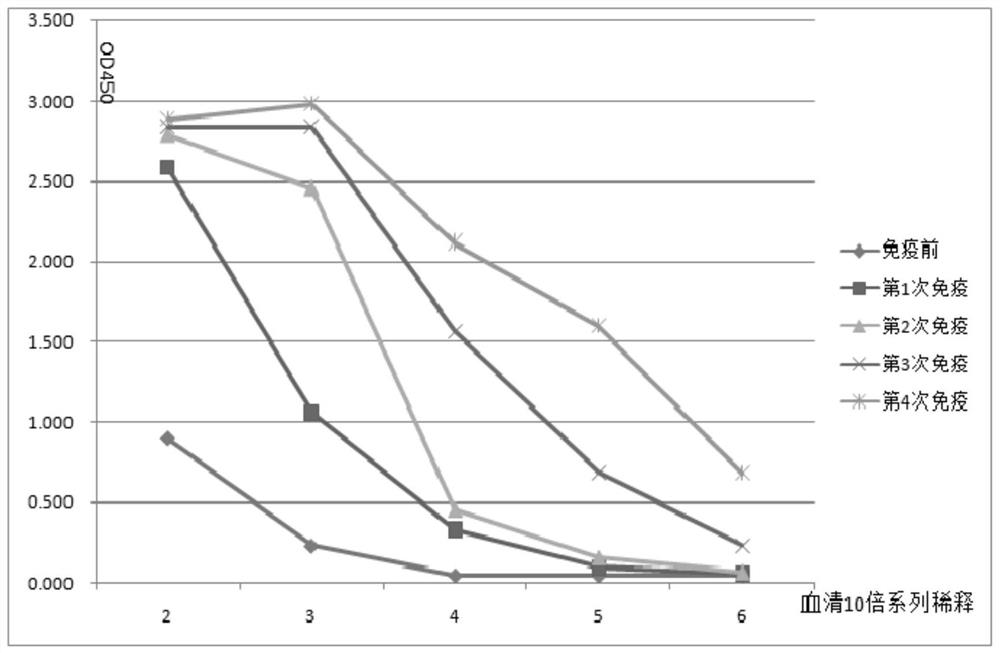
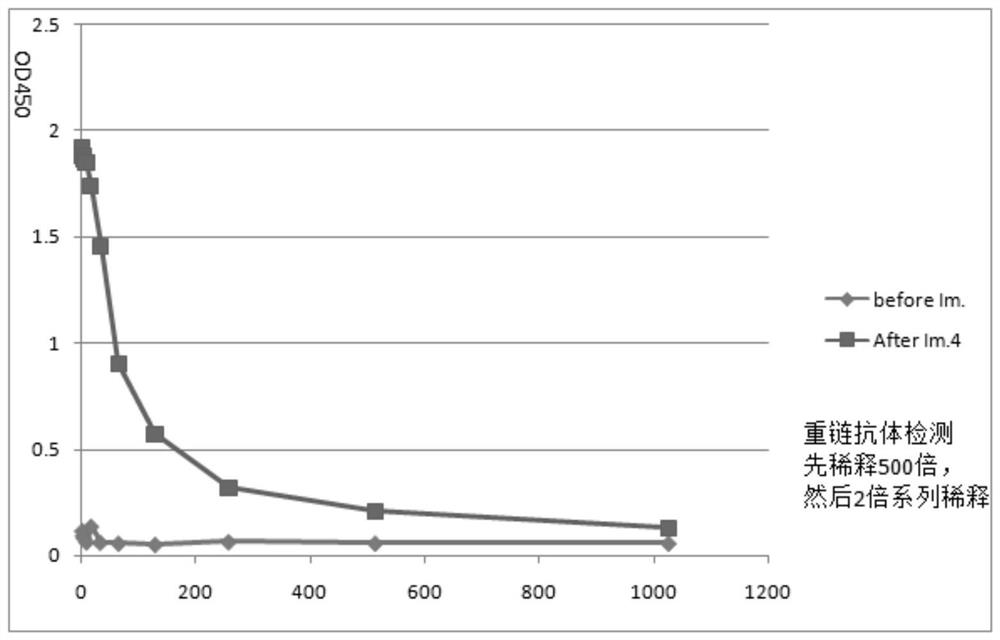
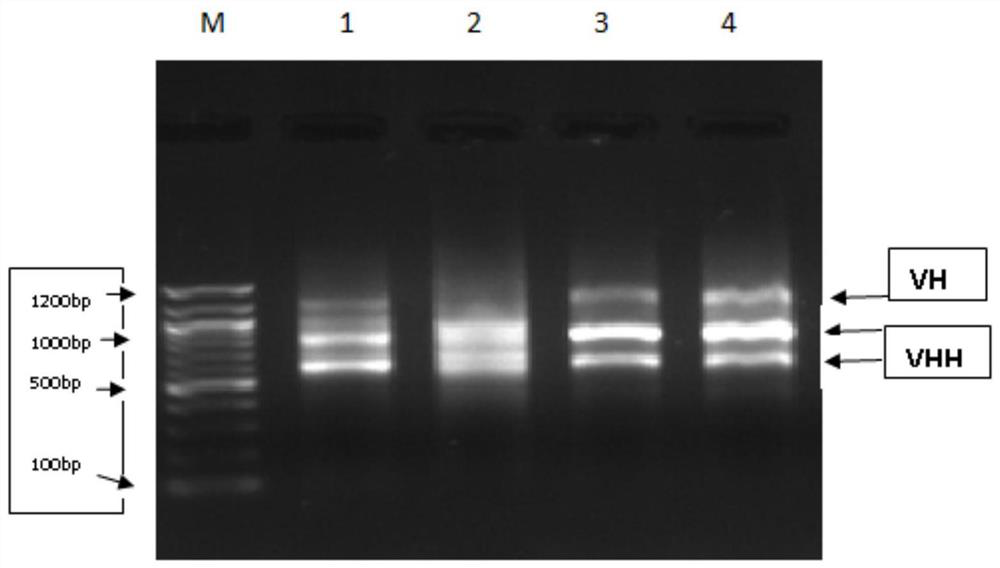
[0072] (2) From the second immunization, peripheral blood was collected one week after each immunization, and ELISA was used to monitor the antibody production level of Ig antigen. The results are shown in figure 1 , the serum titer was the highest after the fourth immunization. Use protein G column chromatography to separate the heavy ...
Embodiment 2
[0105] Example 2 Expression and purification of avian influenza virus nucleoprotein and construction of avian influenza virus nucleoprotein-specific single domain antibody gene library
[0106] 1. Cloning, expression and purification of avian influenza virus nucleoprotein: the gene is synthesized from Translate DNA SequencePet30arc+T7#3.seq (5076,5561), and the gene sequence is (sequence table SEQ ID NO.46):
[0107] 2. Construction of the expression vector: PCR amplified products were separated and recovered by agarose gel electrophoresis, digested with BamHI (BioLab Company), XhoI (BioLab Company), and treated with the same enzyme carrier pET-28a (Sigma) by 3 :1 end ratio for cohesive end ligation. Ligation product by CaCl 2 The transformation method was used to transform competent Escherichia coli JM109 (Sigma), and kanamycin resistance was used to screen recombinant clones. Plasmid DNA of positive clones was extracted for colony PCR. The correct positive recombinants wi...
Embodiment 3
[0111] Example 3 Screening of anti-Ig single domain antibodies
[0112] 1. Screening of anti-IgA specific single domain antibody
[0113] Use 100ug / ml of purified human IgG and IgA to coat the polystyrene microwells with high adsorption capacity of Thermo Electric Company, 150ul / well, overnight at 4°C. Aspirate the coated antigen, add 350ul 2% MPBST (2% skimmed milk, 0.05M, pH7.2-7.4PBS, 0.05% T20), block at room temperature for 2 hours, remove the blocking solution, add the VHH in the above example 1 Phage 5X10 for GenBank 11 , combined at room temperature for 2 hours, rinsed with PBST and PBS 10 times each, added TEA and allowed to stand at room temperature for 10 minutes, eluted specifically bound phage, neutralized TEA with 1M pH8.0 Tris-HCl, and kept on ice. Infect the bacteriophage with semi-logarithmic phase growth TG1, take appropriate amount of bacterial solution and dilute it, spread it on AMP / LB plate, incubate at 32°C, measure the titer of the eluent, and superin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com