In-vitro amplification kit and amplification method for regulatory T cells in human peripheral blood
A technology of human peripheral blood and kits, which is applied in the direction of cell culture active agents, blood/immune system cells, animal cells, etc., and can solve the problems of in vitro function experiments that cannot be carried out smoothly, biological safety that needs to be studied, and effective amplification, etc. problems, to achieve the effect of retaining biological functions, stable phenotype and function, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0034] According to a preferred embodiment of the present invention, the coating antibody is one selected from CD4 monoclonal antibody, CD25 monoclonal antibody and FoxP3 monoclonal antibody, preferably CD4 monoclonal antibody.
[0035] The present inventors have found that the CD4 monoclonal antibody has a wide range of sources, low cost, and is easy to obtain. It is selected as the first coating antibody, which can efficiently and massively expand CD4-positive T cells in peripheral blood mononuclear cells, and can expand the proliferation of Treg cells. The number of basic cells cultured is convenient for further screening and culture operations in the later stage.
[0036] In a further preferred embodiment, the first activating factor is interferon-γ (IFN-γ).
[0037] In the present invention, gamma interferon (IFN-γ) is selected as the activation factor because it can promote the development and differentiation of Treg and negatively regulate the occurrence and development...
Embodiment 1
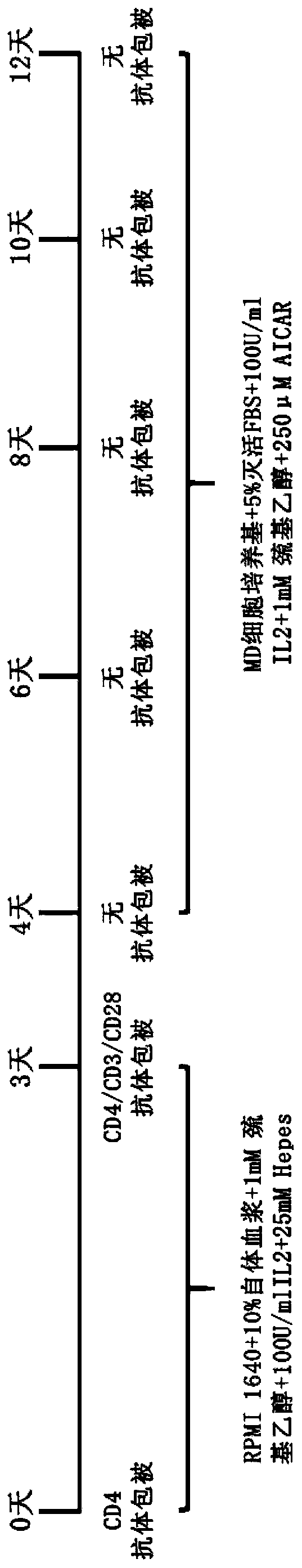
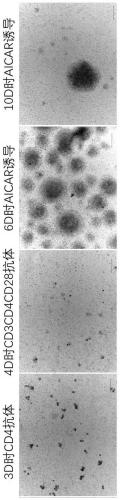
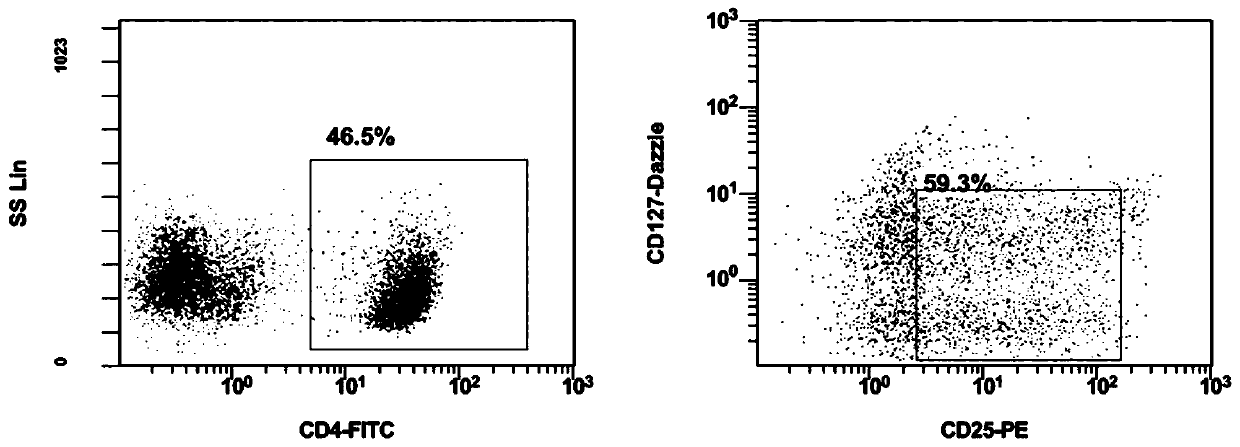
[0157] Such as figure 1 As shown, the in vitro expansion of the human peripheral blood regulatory T cells is carried out according to the following steps:
[0158] Step 1: Take 50-100ml of anticoagulated peripheral blood, centrifuge at 2000rpm for 20min, separate and collect plasma, inactivate at 56°C for 30min, and centrifuge at 1500rpm for 10min to obtain autologous plasma;
[0159] Add 1-1.5 times the plasma volume of sterile PBS buffer to the blood from which the plasma has been removed to dilute the blood, and then use the lymphocyte separation medium Ficoll-Paque (purchased from Beijing Biolab Technology Co., Ltd., product number BTN131136) to separate Peripheral blood mononuclear cells were obtained.
[0160] Step 2: Resuspend the mononuclear cells obtained in step 1 with a selection medium, wherein the selection medium consists of: RPMI 1640 medium + inactivated autologous plasma + β-mercaptoethanol + IL-2 + Hepes, wherein , the final concentration of inactivated aut...
Embodiment 2
[0169] The method used in this example is similar to Example 1, except that in step 2, the final concentration of the CD4 monoclonal antibody is 22 μg / ml, and the final concentration of IFN-γ is 1500 U.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Final concentration | aaaaa | aaaaa |
| Final concentration | aaaaa | aaaaa |
| Final concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com