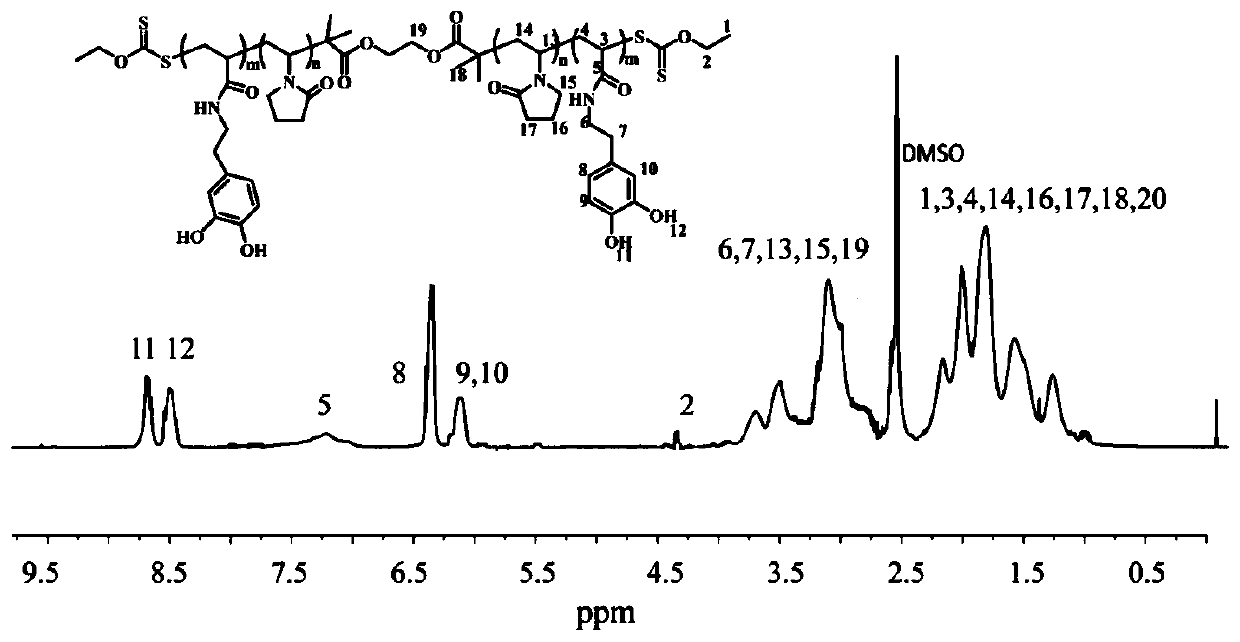
PVP compound with terminal containing dithioester, topological polymer, and preparation methods for PVP compound with terminal containing dithioester and topological polymer
A technology of dithioesters and compounds, which is applied in the field of synthesis of antifouling compounds, can solve problems such as difficulty in realization, difficulty in large-area use, and difficulty in scale-up, and achieve the effects of excellent adhesion and excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] For the second solution of the present invention, the present invention provides a kind of preparation method of the PVP compound containing dithioester at the end, comprising the following steps:
[0036] Compound I is reacted with α-bromoisobutyryl bromide from a compound containing a hydroxyl group at the end, compound I is reacted with potassium ethyl xanthate to obtain compound II, and compound II reacts with N-ethylene under the action of the first initiator Pyrrolidone reaction to obtain a PVP compound containing a dithioester at the end;
[0037] Among them, the compound having a hydroxyl group at the terminal is a monohydric alcohol or a polyhydric alcohol.
[0038] The preparation method of the PVP compound containing a dithioester at the terminal provided in the second solution of the present invention is used to prepare the PVP compound containing a dithioester at the terminal provided in the first solution of the present invention.
[0039] Specifically, c...
Embodiment 1
[0080] The preparation of the PVP compound containing dithioester at the end:
[0081]
[0082] Add ethylene glycol ((2.0g, 32.3mmol), anhydrous tetrahydrofuran (50mL) and triethylamine (10.7mL) into the reaction kettle, stir well and add α-bromoisobutyryl bromide (17.8g, 77.28mmol ), after stirring at room temperature for 12h, slowly add diluted hydrochloric acid solution (10wt%) (10mL).The organic phase was washed with aqueous sodium bicarbonate solution (5wt%), and then concentrated to obtain the crude product after drying over anhydrous magnesium sulfate, and the crude product was washed with methanol Recrystallize twice to obtain EG-Br.
[0083] Weigh EG-Br (0.72g, 2mmol), potassium ethyl xanthate (1.92g, 12mmol) and absolute ethanol (20mL) in a reaction flask, and stir the reaction at room temperature for 16h. Filtration, after the filtrate was diluted with dichloromethane, washed three times with deionized water (50mL), the organic phase was dried and concentrated c...
Embodiment 2
[0089] The preparation of the PVP compound containing dithioester at the end:
[0090] Referring to the synthetic route of the PVP compound with dithioester at the end of the linear structure provided in Example 1, the molar ratio of EG-CTA to N-vinylpyrrolidone is selected as 1:400, and the dithioester at the end of the linear structure is synthesized PVP compound (L-PVP) (n=200).
[0091] Preparation of topological polymers:
[0092] In the reaction bottle, add the PVP compound (L-PVP) (n=200) (13.45g, 0.3mmol) containing dithioester at the end of the linear structure, azobisisobutylcyanide (AIBN) (16.4mg, 0.3mmol ), a polymerizable catechol monomer DMA (5.10 g, 24.6 mmol) and dimethylformamide (20 mL), reacted at 60 ° C for 6 h under the protection of argon. The reaction system was lowered to room temperature, precipitated in diethyl ether, filtered, the precipitate was washed twice with hot methanol, and the obtained solid was vacuum-dried to obtain a topological polymer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com