Preparation method of quaternary visible light catalysis nano composite material
A nano-composite material, visible light technology, applied in the field of photocatalytic materials, can solve the problem of low photocatalytic efficiency, and achieve the effect of improving the reaction rate, accelerating crystal crystallization, and prolonging the life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A method for preparing a quaternary visible light-catalyzed nanocomposite material, specifically comprising the following steps:
[0030] (1) Evenly disperse copper oxide and zinc sulfide with a mass ratio of 1:1~4 in deionized water to form a mixed solution;
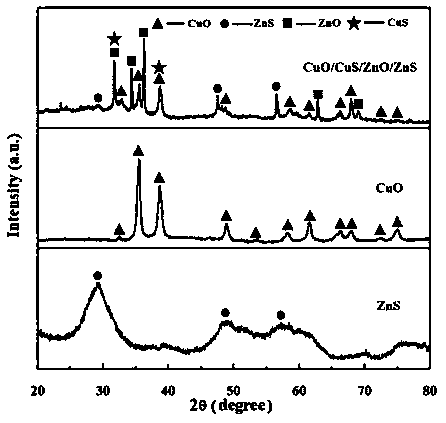
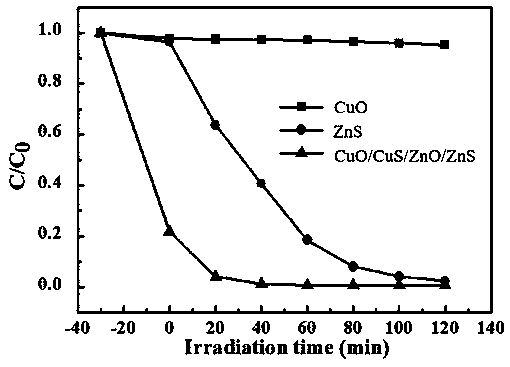
[0031] (2) Put the mixed solution obtained in step (1) in a microwave reactor, react with microwave irradiation, cool to room temperature, filter, wash and dry to obtain CuO / CuS / ZnO / ZnS quaternary visible photocatalytic nanocomposites.
[0032]Here, copper oxide is dispersed first, and then zinc sulfide is added for dispersion, because copper oxide is in the form of flakes, rods, wires, etc., while zinc sulfide has a spherical structure. Relatively speaking, copper oxide is difficult to disperse, and sulfide Zinc is easy to disperse, so copper oxide is added first, and then zinc sulfide is added, which can effectively ensure that zinc sulfide is fully mixed with copper oxide, and avoid copper oxide dispersion th...
Embodiment 1
[0052] (1) Preparation of zinc sulfide: Dissolve 0.2195g of zinc acetate and 0.3005g of thioacetamide in 40mL of deionized water to prepare precursor solution A. The molar ratio of zinc acetate to thioacetamide is 1:4, and then Precursor solution A was placed in a microwave reactor and irradiated at a power of 130W for 9 minutes. After the reaction was completed, it was cooled to room temperature and left to stand overnight. The precipitate was filtered, washed three times with deionized water and absolute ethanol, and dried for 12 hours. That is, white ZnS is obtained;
[0053] (2) Preparation of copper oxide: Dissolve 0.2500g copper sulfate and 0.1600g sodium hydroxide in 40mL deionized water to prepare precursor solution B. The molar ratio of copper sulfate and sodium hydroxide is 1:4, and then the precursor solution B is placed in a microwave reactor, set the power to 260W, and irradiated by microwave for 12min. After the reaction, cool naturally to room temperature, filte...
Embodiment 2
[0059] (1) Preparation of zinc sulfide: Dissolve 0.2195g of zinc acetate and 0.2254g of thioacetamide in 40mL of deionized water to prepare precursor solution A. The molar ratio of zinc acetate to thioacetamide is 1:3, and then Precursor solution A was placed in a microwave reactor and irradiated at a power of 390W for 5 minutes. After the reaction was completed, it was cooled to room temperature and left to stand overnight. The precipitate was filtered, washed three times with deionized water and absolute ethanol, and dried for 12 hours. White ZnS can be obtained;
[0060] (2) Preparation of copper oxide: Dissolve 0.2500g of copper sulfate and 0.1200g of sodium hydroxide in 40mL of deionized water to prepare precursor solution B. The molar ratio of copper sulfate and sodium hydroxide is 1:3, and then the precursor solution B is placed in a microwave reactor, set the power to 130W, irradiated for 15min, naturally cooled to room temperature after the reaction, filtered the blac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com