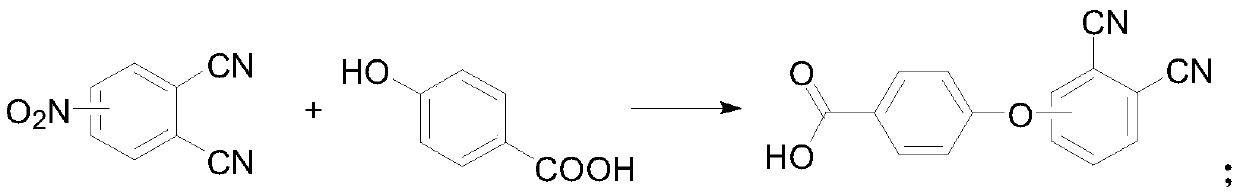Organic-inorganic composite nanometer photocatalyst and preparation method thereof
A nano-photocatalyst, inorganic composite technology, applied in organic compound/hydride/coordination complex catalysts, inorganic chemistry, chemical instruments and methods, etc., can solve the problem of poor solubility, unsatisfactory catalytic performance, and difficulty in uniform dispersion problem, to achieve the effect of improving solubility, improving solubility, and increasing dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Zinc Phthalocyanine
[0033] Under the protection of nitrogen, add 50 mL of dimethyl sulfoxide to the reaction flask, add 4 g of 4-nitrophthalonitrile and 7 g of p-hydroxybenzoic acid under stirring, then add 15 g of potassium carbonate, and stir at 30 ° C for 24 h , then add the reaction solution dropwise to 150mL of deionized water, adjust the pH value to 1 with concentrated hydrochloric acid, a flocculent precipitate precipitates, separate the precipitate by suction filtration, wash with deionized water, recrystallize with methanol, and then place at 50°C Dried in a vacuum oven to obtain 4-(4-carboxyphenoxy)phthalonitrile;
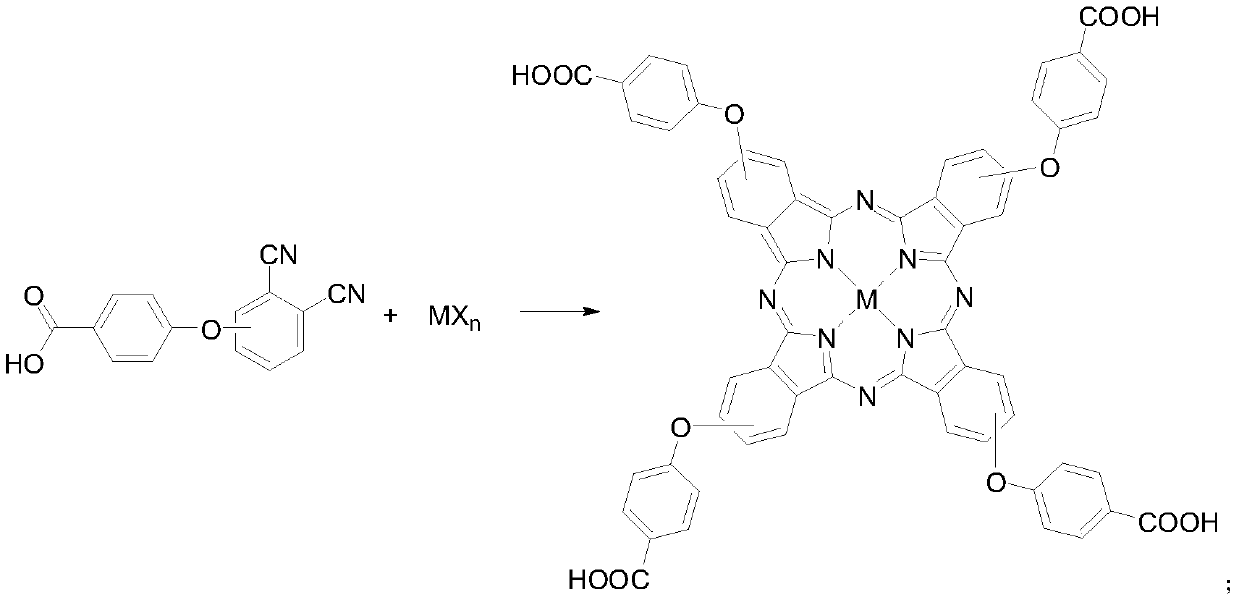
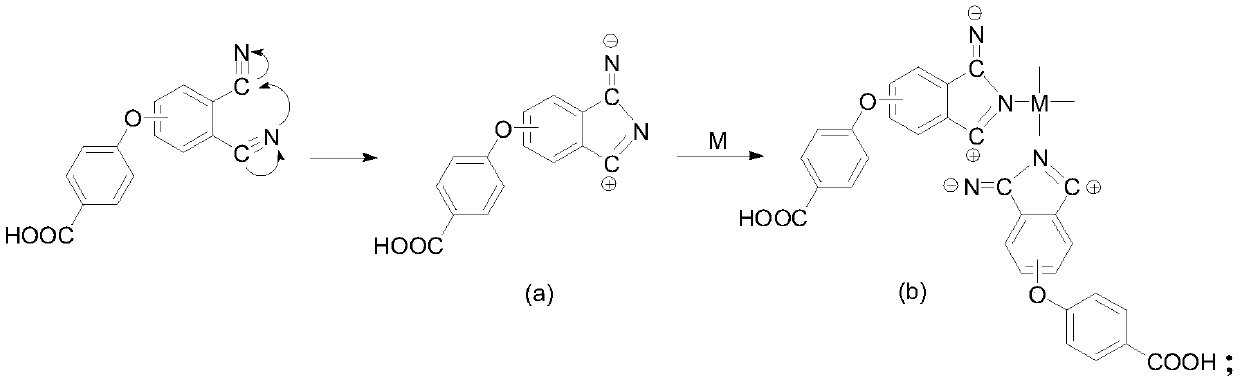
[0034] Under the protection of nitrogen, dissolve 2g of 4-(4-carboxyphenoxy)phthalonitrile and 0.8g of zinc chloride in 30mL of n-pentanol, heat up to 50°C and add 1,8-diazabicyclo Undec-7-ene 1.6mL, heated to 130°C and reacted for 6h, then cooled to room temperature, added hydrochloric acid to the reaction solution to adjust the ...
Embodiment 2
[0036] Preparation of cobalt phthalocyanine
[0037] Under the protection of nitrogen, add 50mL of dimethyl sulfoxide to the reaction flask, and add 3g of 4-nitrophthalonitrile and 7g of p-hydroxybenzoic acid under stirring, then add 12g of potassium carbonate, and stir at 30°C for 24h , then add the reaction solution dropwise to 150mL of deionized water, adjust the pH value to 1 with concentrated hydrochloric acid, a flocculent precipitate precipitates, separate the precipitate by suction filtration, wash with deionized water, recrystallize with methanol, and then place at 50°C Dried in a vacuum oven to obtain 4-(4-carboxyphenoxy)phthalonitrile;
[0038] Under the protection of nitrogen, dissolve 2g of 4-(4-carboxyphenoxy)phthalonitrile and 1g of cobalt acetate in 30mL of n-pentanol, and add 1,8-diazabicycloundecone after heating up to 50°C Carb-7-ene 1.6mL, heated to 130°C and reacted for 6h, cooled to room temperature, added hydrochloric acid to the reaction solution to ad...
Embodiment 3
[0040] Preparation of copper phthalocyanine
[0041] Under nitrogen protection, add 50 mL of dimethyl sulfoxide to the reaction flask, add 4 g of 3-nitrophthalonitrile and 7 g of p-hydroxybenzoic acid under stirring, then add 14 g of potassium carbonate, and stir for 24 hours at 30°C , then add the reaction solution dropwise to 150mL of deionized water, adjust the pH value to 1 with concentrated hydrochloric acid, a flocculent precipitate precipitates, separate the precipitate by suction filtration, wash with deionized water, recrystallize with methanol, and then place at 50°C Dried in a vacuum oven to obtain 4-(4-carboxyphenoxy)phthalonitrile;
[0042] Under nitrogen protection, dissolve 2 g of 4-(4-carboxyphenoxy)phthalonitrile and 0.7 g of cuprous chloride in 30 mL of n-pentanol, and add 1,8-diazabis Cycloundec-7-ene 1.6mL, heated to 130°C and reacted for 6h, cooled to room temperature, added hydrochloric acid to the reaction solution to adjust the pH to 1, a large amount ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com