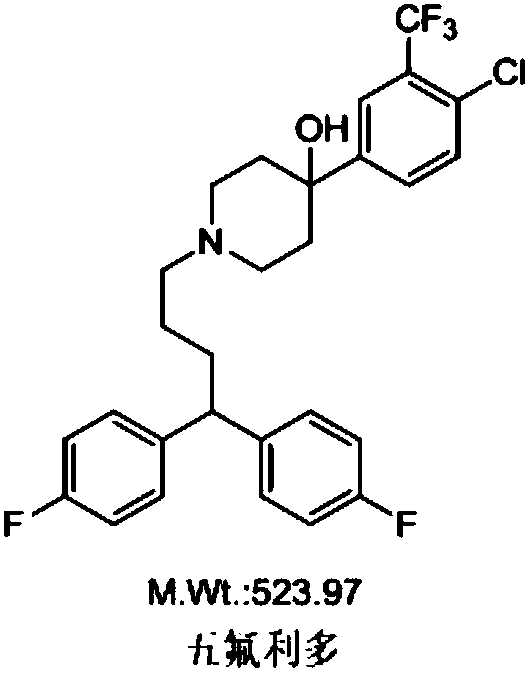
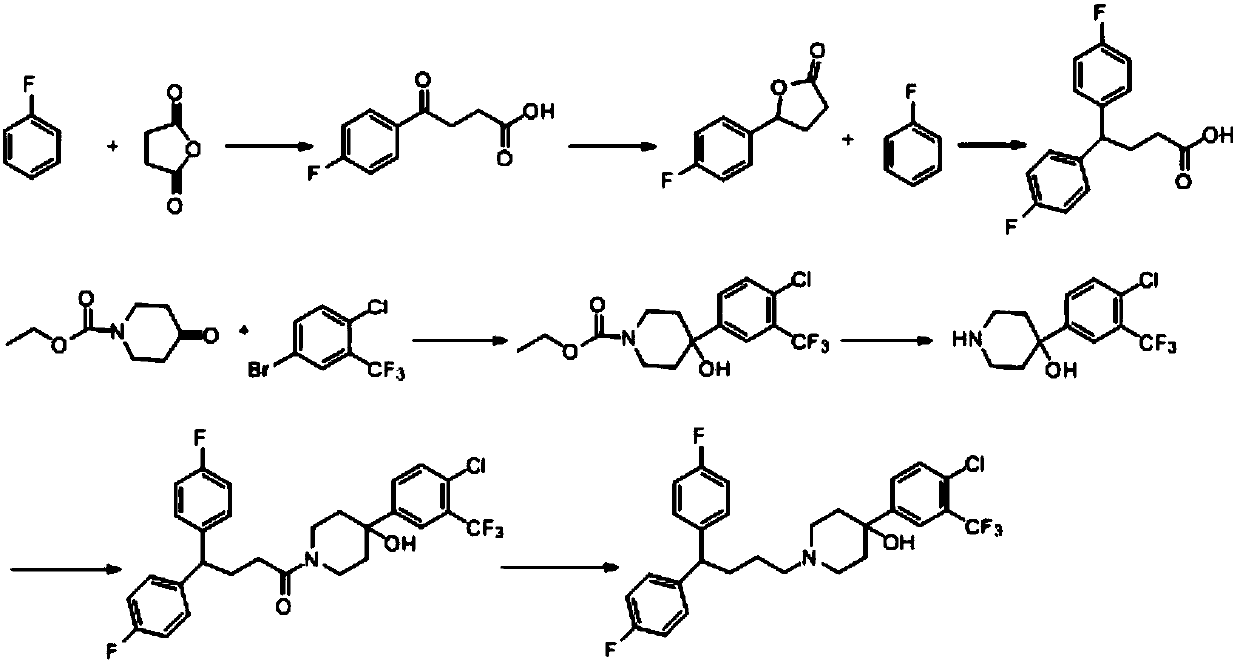
Improved method for preparing penfuridol
A technology of pentafluridol and fluorophenyl, which is applied in the field of preparation of pentafluridol, can solve the problems of many impurities and low product purity, and achieve the effects of good purity, high yield and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
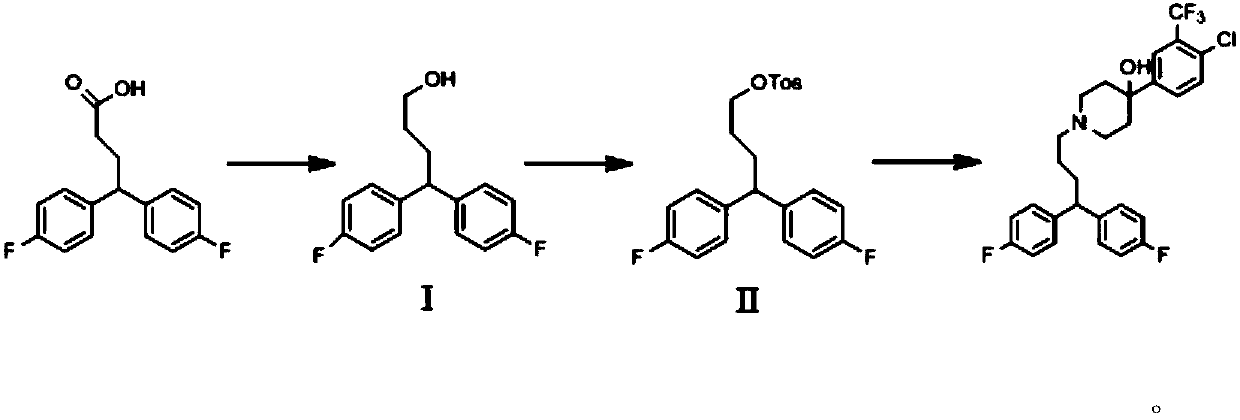
[0049] (1) Synthesis of intermediate Ⅰ
[0050]Put 20 g (0.072 moles) of 4,4-bis(4-fluorophenyl) butanoic acid into a three-necked flask, stir and dissolve 172 g (2.00 moles) of methyl tetrahydrofuran, add 5.4 g (0.039 moles) of potassium carbonate, and control the temperature Below 10°C, slowly add 2.89g (0.076 mol) of lithium aluminum tetrahydrogen, and keep stirring at about 0-10°C for 1-2h. After the reaction, the temperature was controlled below 10°C, and 2.9g (0.161 mol) of water, 2.9g (0.011 mol) of 15% sodium hydroxide solution (mass fraction) and 8.7g (0.483 mol) of water were added dropwise in sequence. , naturally heated to 25°C-30°C for filtration, the filter cake was washed with methyl tetrahydrofuran, the combined filtrates were concentrated to dryness to obtain 17.5g of the product 4,4-bis(4-fluorophenyl)-1-butanol (yield 92.6%).
[0051] The general reaction formula is as follows:
[0052]
[0053] (2) Synthesis of Intermediate II
[0054] Under nitrogen...
Embodiment 2
[0062] (1) Synthesis of intermediate Ⅰ
[0063] Put 20 g (0.077 moles) of 4,4-bis(4-fluorophenyl) butanoic acid into a three-necked flask, stir and dissolve 172 g (2.38 moles) of tetrahydrofuran, add 5.4 g (0.052 moles) of neutral alumina, and control the temperature Below 10°C, slowly add 2.00g (0.072 mol) of diborane, keep warm at 0-20°C and stir for 2-3h. After the reaction was completed, the temperature was controlled below 10°C, and 2.9g (0.016 moles) of water, 2.9g (0.011 moles) of 15% sodium hydroxide solution, and 8.7g (0.483 moles) of water were added dropwise successively. After quenching, stir and naturally Raise the temperature to 25°C-30°C for filtration, wash the filter cake with tetrahydrofuran, combine the filtrates, and concentrate to dryness to obtain 17 g of 4,4-bis(4-fluorophenyl)-1-butanol (yield 84.1%).
[0064] (2) Synthesis of Intermediate II
[0065] Under nitrogen protection, get 9g (0.034 moles) of 4,4-bis(4-fluorophenyl)-1-butanol obtained in step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com