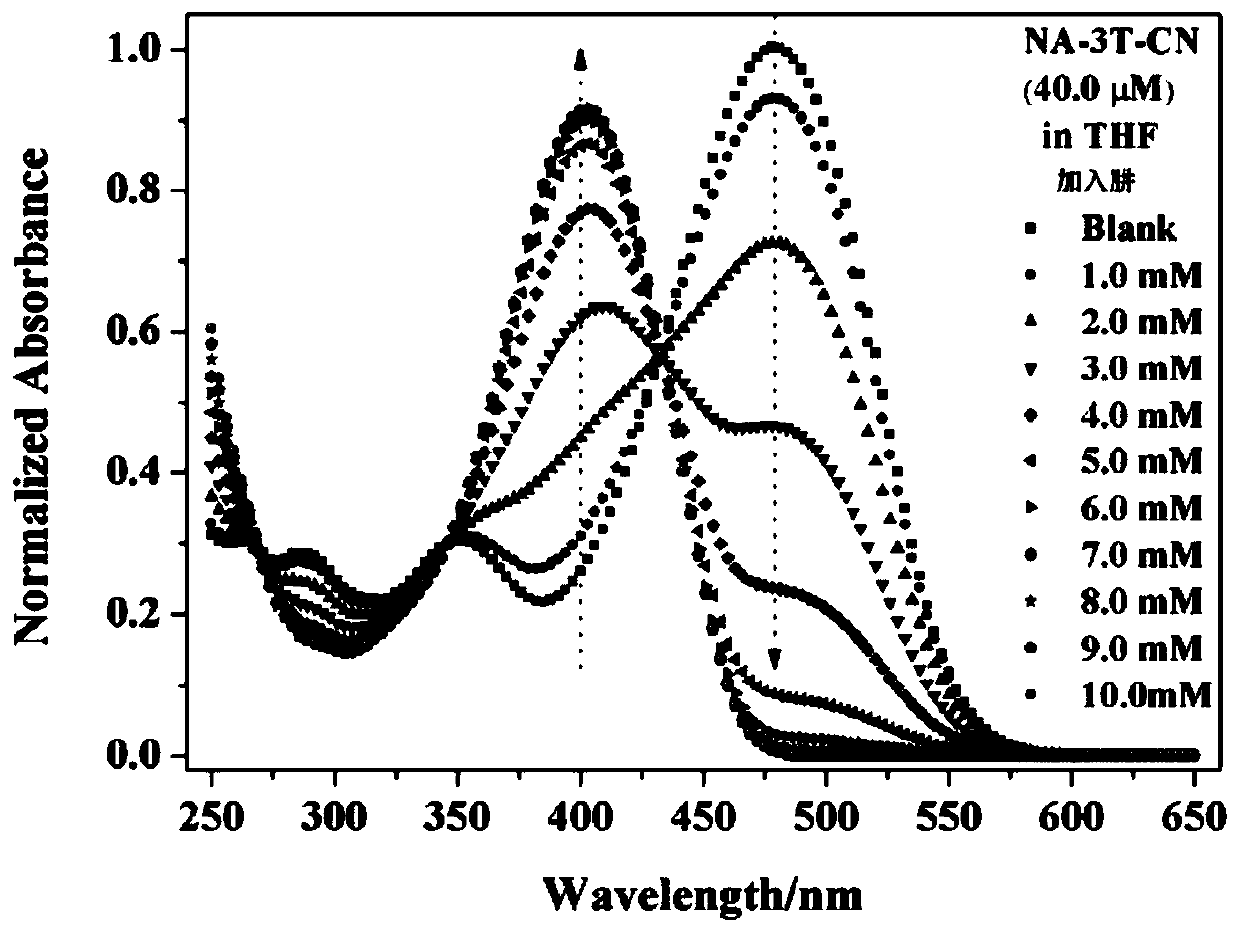
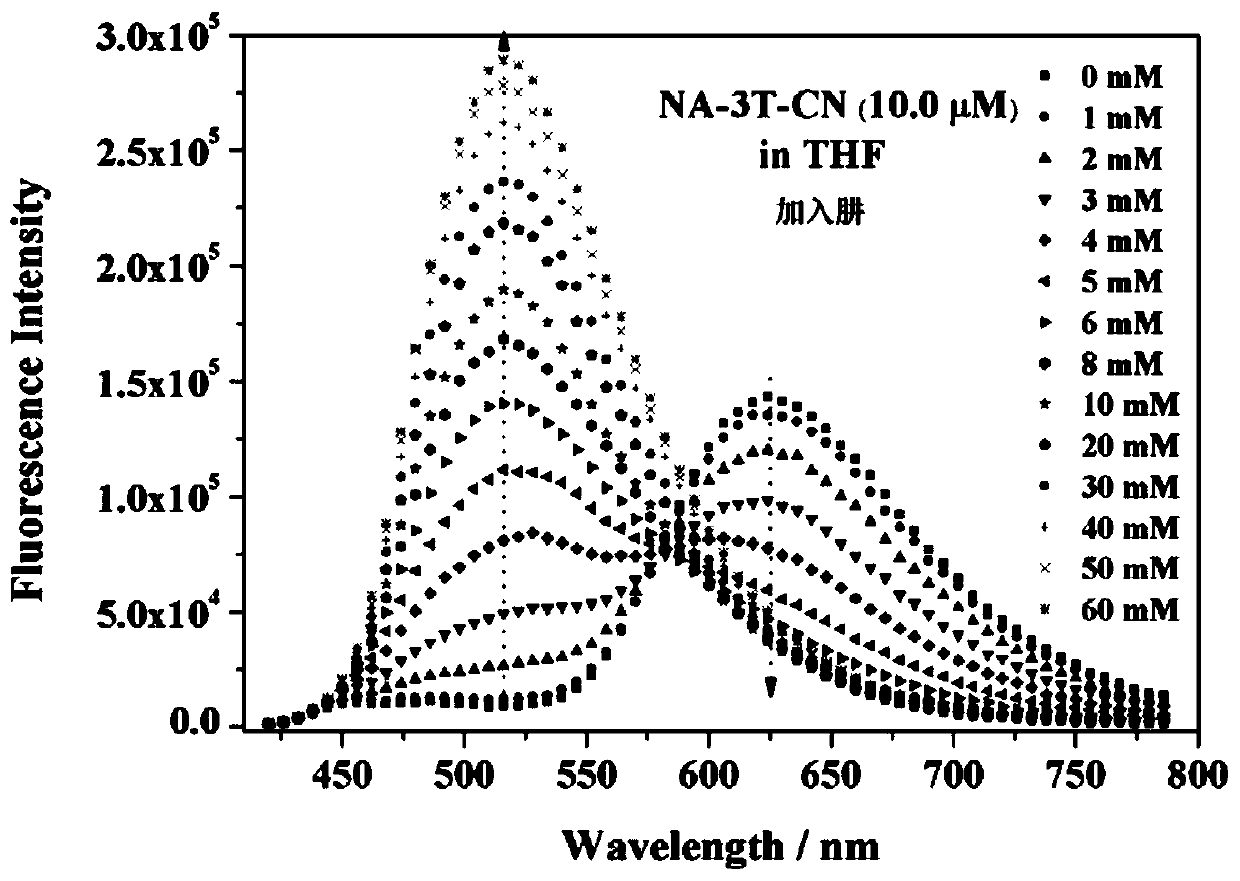
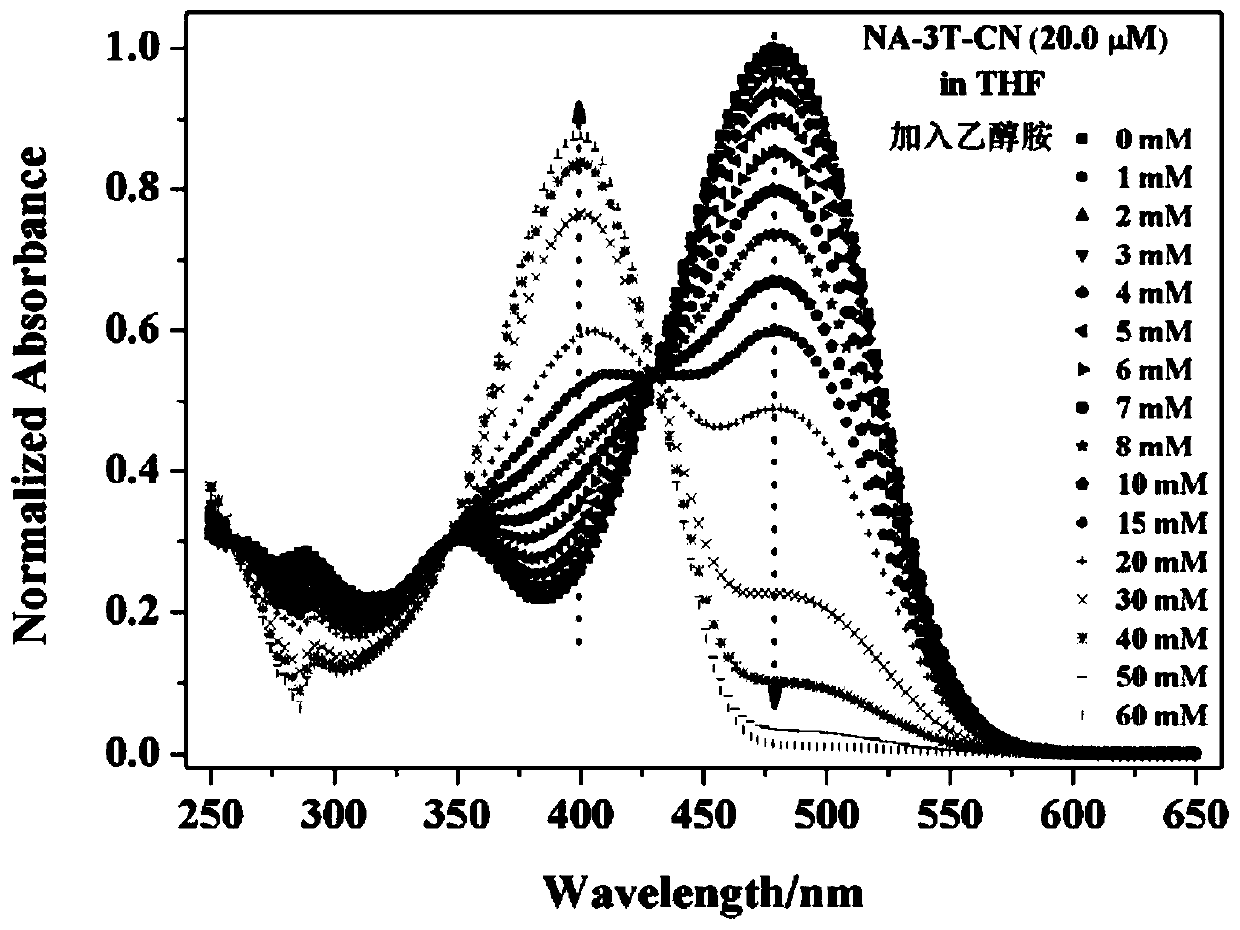
Dual-mode sensor array and application thereof in distinguishing and identifying hydrazine and organic amine
A sensor array and dual-mode technology, which is applied in the field of small molecule fluorescence sensing, can solve the problems of discrimination and fewer high-performance fluorescent probes, and achieve the effects of smaller energy gap value, improved photoelectric properties, and enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Preparation of dicyanovinyl functionalized oligothiophene derivatives (NA-3T-CN)
[0050] (1) Synthesis of compound 1
[0051] Under nitrogen protection, dark, 0 ℃ and stirring conditions, 3.2g (17.8mmol) N-bromosuccinimide (NBS) was added dropwise to 45mL containing 5.0g (20.0mmol) 2,2′:5 ′,2″-trithiophene in N,N-dimethylformamide (DMF) solution, react at room temperature for 24 hours, after the reaction is completed, pour the reactant into ice water, extract with 100mL dichloromethane, and wash the organic phase with water 3 times, dried over anhydrous sodium sulfate, and rotary evaporated to obtain light yellow solid compound 1, the reaction equation is as follows:
[0052]
[0053] (2) Synthesis of compound 2-1
[0054] Under the protection of nitrogen, add 0.24g (10.0mmol) new magnesium chips and 0.05g initiator iodine to the three-necked flask equipped with a reflux condenser and a constant pressure dropping funnel, then add 40mL of dissolved 1.54 mL (11....
Embodiment 2
[0076] 1. Preparation of dicyanovinyl functionalized oligothiophene derivatives (3T-CN)
[0077] (1) Synthesis of compound 3-2
[0078] Under nitrogen protection, 0°C and stirring conditions, 1.2mL (13.0mmol) of phosphorus oxychloride was slowly added dropwise to 60mL of 2.48g (10.0mmol) of 2,2′:5′,2″-trithiophene After the dropwise addition, heat the reaction solution to 60°C and continue the reaction for 4 hours. After cooling, pour the above reaction solution into 100mL saturated aqueous sodium acetate solution, filter under reduced pressure to obtain a red The solid was washed several times with deionized water, and the crude product was separated by column chromatography using dichloromethane as a developing solvent to obtain red solid compound 3-2 (referred to as 3T-CHO).
[0079] Its reaction equation is as follows:
[0080]
[0081] (2) Synthesis of dicyanovinyl functionalized oligothiophene derivatives
[0082] Add 0.27g (1.0mmol) of compound 3-2, 0.075mL (1.2mm...
Embodiment 3
[0088] 1. Preparation of dicyanovinyl functionalized oligothiophene derivatives (4T-CN)
[0089] (1) Synthesis of Compound 2-1
[0090] Under the protection of nitrogen, add 0.24g (10.0mmol) new magnesium chips and 0.05g initiator iodine to the three-necked flask equipped with a reflux condenser and a constant pressure dropping funnel, then add 40mL of dissolved 1.54 mL (11.0 mmol) of 1-bromothiophene in tetrahydrofuran was heated to 80° C. and reacted until the magnesium dust disappeared completely to obtain the prepared Grignard reagent.
[0091] Under nitrogen protection, weigh 3.62g (8.0mmol) of compound 1, 0.22g (0.4mmol) of 1,3-bis(diphenylphosphinopropane)nickel dichloride catalyst into a three-necked flask, add 40mL of tetrahydrofuran, and Add the above-prepared Grignard reagent dropwise at 0°C, slowly raise the temperature under stirring, and reflux for 24 hours; after the reaction is completed, cool to room temperature, pour the reaction solution into 150 mL of satu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com