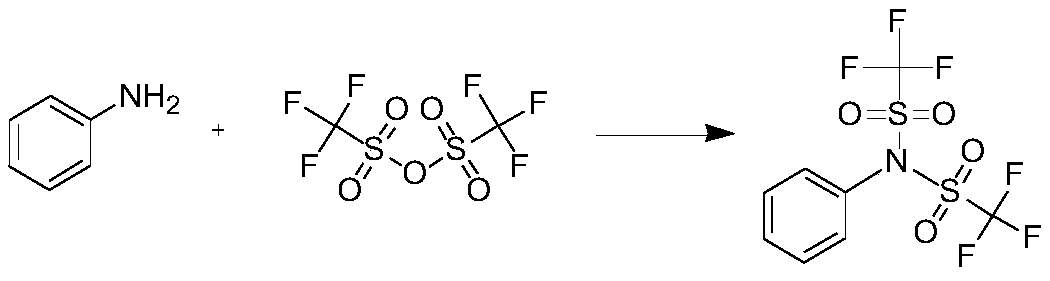
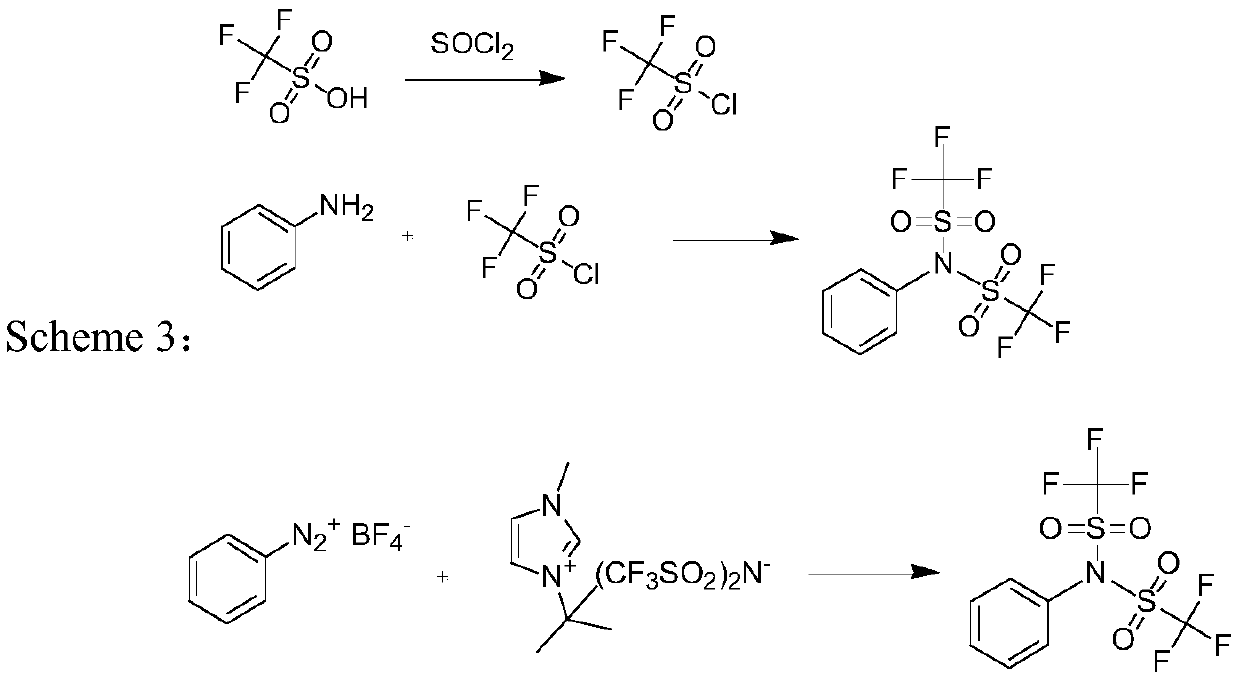
Preparation method of N-phenyl bis (trifluoromethanesulfonyl) imide
A technology of phenylbistrifluoromethanesulfonimide and phenyltrifluoromethanesulfonamide, which is applied in the field of preparation of N-phenylbistrifluoromethanesulfonimide, and can solve the problem that the reaction product has many impurities and high cost , high price of trifluoromethanesulfonic acid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 9.3 kg of aniline, 40 L of acetonitrile, and 13 kg of triethylenediamine into a 100L stainless steel reactor, and connect the trifluoromethanesulfonyl fluoride steel cylinder; evacuate the reactor to -0.09Mpa, and feed 20 kg of trifluoromethanesulfonyl fluoride Gas, seal the reactor; stir and react at 40°C for 8 hours, observe that the pressure of the reactor gradually decreases from 0.23Mpa to 0.08Mpa, and maintains no significant change within half an hour.
[0049] Open the acyl fluoride recovery pipeline, reclaim excessive acyl fluoride, after the reactor pressure is reduced to 0Mpa, close the acyl fluoride recovery pipeline. Open the distillation system, and distill off 34L of acetonitrile. Add 40L dichloromethane in reactor again, 0.4 kilogram of 4-dimethylaminopyridine, pass into 20 kilograms of trifluoromethanesulfonyl fluoride again, closed reaction 15 hours, reactor pressure is reduced to 0.06Mpa by 0.21Mpa.
[0050] Recover excess trifluoromethanesulfony...
Embodiment 2
[0052] Add 9.3 kg of aniline, 35 L of dimethylformamide, and 33 kg of DBU into a 100 L stainless steel reactor, and connect the trifluoromethanesulfonyl fluoride steel cylinder. Reactor is evacuated to-0.09Mpa, feeds 22 kilograms of trifluoromethanesulfonyl fluoride gas, and seals reactor. Stirring and reacting at 60°C for 8 hours, the pressure of the reactor gradually decreased from 0.29Mpa to 0.09Mpa, and there was no obvious change within half an hour. Open the acyl fluoride recovery pipeline, reclaim excessive acyl fluoride, after the reactor pressure is reduced to 0Mpa, close the acyl fluoride recovery pipeline. Open distillation system, distill and remove 30L dimethylformamide. Then add 50L of toluene, 0.3 kg of 4-dimethylaminopyridine, pass into 25 kg of trifluoromethanesulfonyl fluoride again, close the reaction at 60 ° C for 10 hours, the pressure of the reactor is reduced from 0.26Mpa to 0.1Mpa, and recover the excess trifluoromethane Sulfonyl fluoride, 38L of solv...
Embodiment 3
[0054] Add 9.3 kg of aniline, 45 L of acetonitrile, and 15 kg of triethylenediamine into a 100 L stainless steel reactor, and connect the trifluoromethanesulfonyl fluoride steel cylinder. Reactor is evacuated to-0.09Mpa, feeds 30 kilograms of trifluoromethanesulfonyl fluoride gas, and seals reactor. Stirring and reacting at room temperature for 8 hours, the pressure of the reactor gradually decreased from 0.25Mpa to 0.1Mpa, and there was no obvious change within half an hour. Open the acyl fluoride recovery pipeline, reclaim excessive acyl fluoride, after the reactor pressure is reduced to 0Mpa, close the acyl fluoride recovery pipeline. Open distillation system, remove 40L acetonitrile by distillation. Then add 45L dichloromethane, 0.4 kilograms of 4-dimethylaminopyridine, pass into 23 kilograms of trifluoromethanesulfonyl fluoride again, seal reaction 16 hours, reactor pressure is reduced to 0.06Mpa by 0.22Mpa, reclaim excessive trifluoromethane Sulfonyl fluoride, 40L of so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com