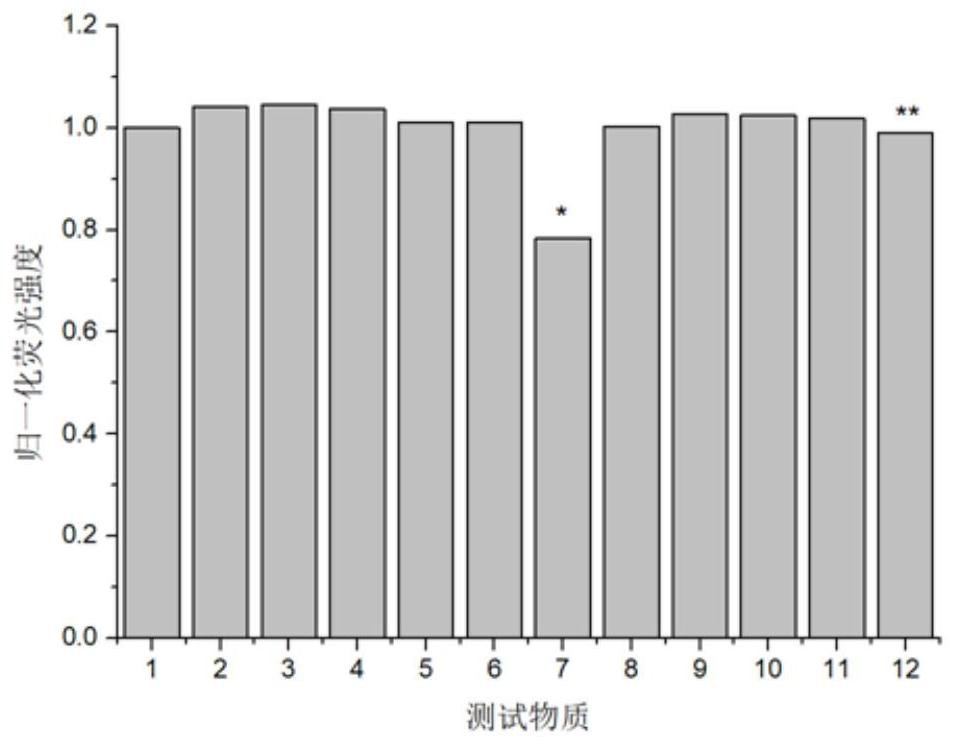
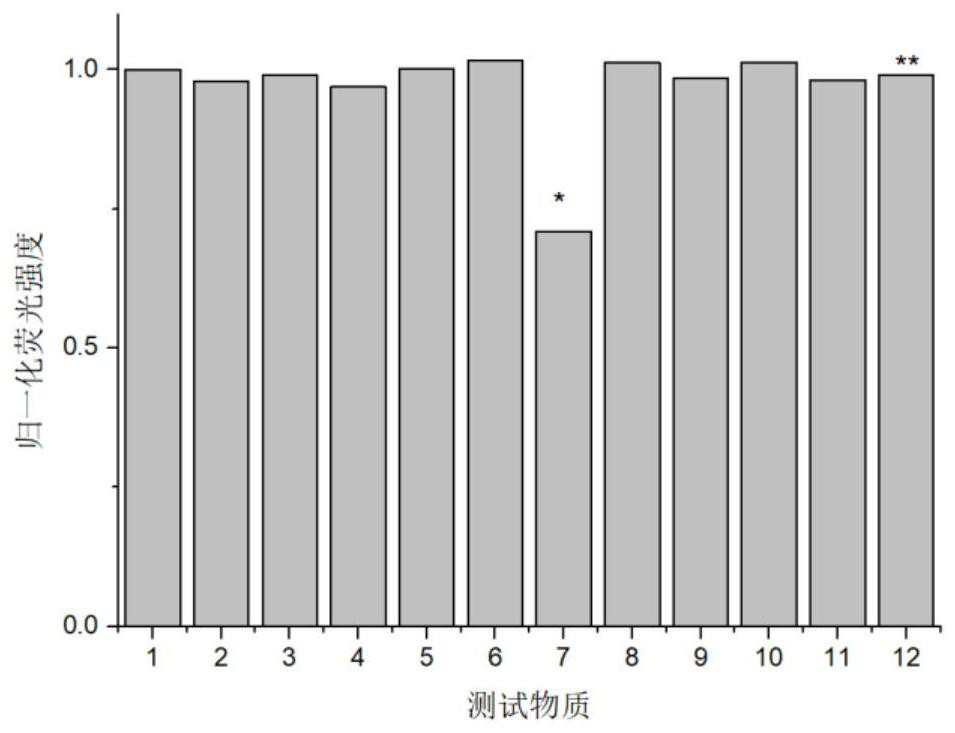
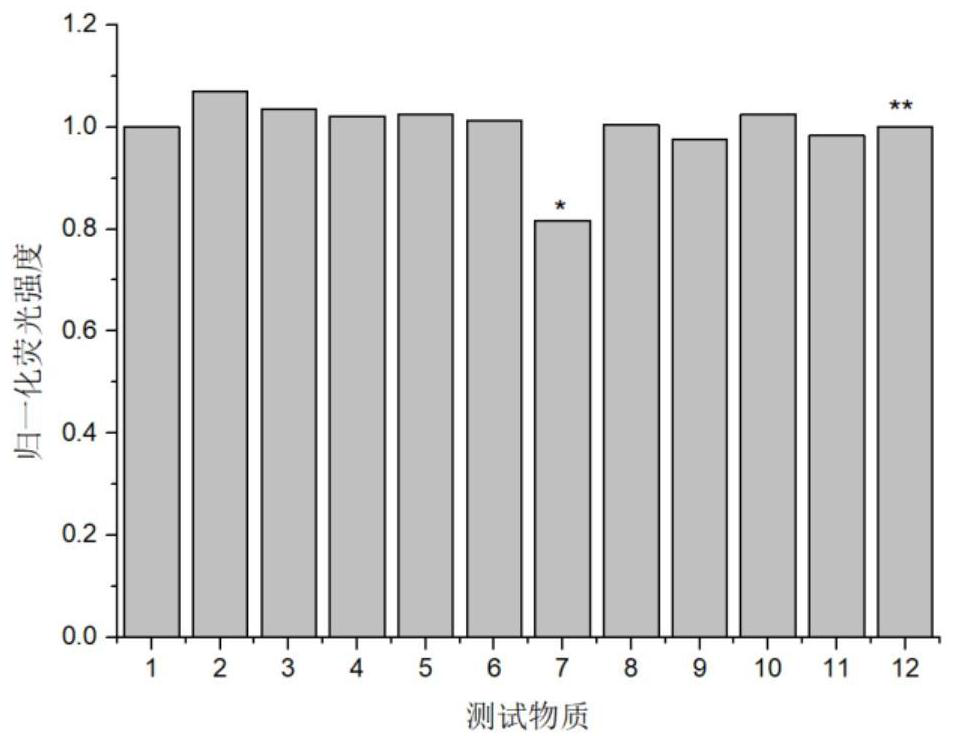
Sulfhydryl-containing fluorescent compound or its thioester derivatives and its preparation and application
A technology of fluorescent compounds and derivatives, which is applied in the preparation of thiols, compounds containing Group 3/13 elements of the periodic table, fluorescence/phosphorescence, etc. It can solve the problems of irreversible detection, inappropriate NO dynamic detection, etc. Selectivity, good biofilm permeability, high detection signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of I-1
[0033]
[0034] Compound 1a is reacted with cystamine in dry dichloromethane under the condition of peptide synthesis condensing agent (such as HBTU). After the end, add ethyl acetate to dilute, wash twice with saturated aqueous sodium bicarbonate solution, saturated aqueous ammonium chloride solution, and saturated saline, and dry the organic phase with anhydrous sodium sulfate, spin dry, and wash with methanol-containing chloroform solution (1 %-10% methanol, gradient elution) as the eluent for silica gel column chromatography purification to obtain 1b (82%), high resolution mass spectrum HRMS: 547.2113 (M+H) + .
[0035] Intermediate 1b was redissolved in methanol, dithiothreitol was dissolved in water, added to the system, and reacted at room temperature. Thin-layer chromatography monitors the reaction. After the end, the methanol is spun off, extracted twice with dichloromethane, the organic layers are combined, dried ove...
Embodiment 2
[0036] Embodiment 2: the preparation of I-2
[0037]
[0038] Add triethylamine to compound 2a and cystamine in dry dichloromethane, and stir at room temperature. After the reaction was completed, pure water and saturated brine were added to wash twice, the organic phase was dried over anhydrous sodium sulfate, spin-dried, and purified by column chromatography to obtain 2b (93%). Mass Spectrum m / z 618.15(M+H) + .
[0039] Referring to the synthesis of compound I-1, 2b was reduced to prepare I-2 with a yield of 80%. High resolution mass spectrum 311.0935(M+H) + .
Embodiment 3
[0040] Embodiment 3: the preparation of I-3
[0041]
[0042] Compound 3a was dissolved in ethanol, added cystamine, and refluxed. After the reaction was complete, it was cooled and filtered with suction to obtain 3b as a yellow solid. Yield 60%. Mass Spectrum m / z 621.20 (M+Na) + .
[0043] Referring to the synthesis of compound I-1, I-3 was prepared by reducing 3b with a yield of 85%. High resolution mass spectrum 301.0989(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com