A kind of garnet type composite electrolyte material and its preparation method and application
A composite electrolyte, garnet-type technology, applied in the field of garnet-type composite electrolyte materials and their preparation, can solve the problem that the garnet-type oxide electrolyte material has not been proposed, is not suitable for large-sized ceramic solid electrolyte materials, and the temperature of the sintering equipment cavity Uneven distribution and other problems, to achieve the effect of low equipment requirements, mild conditions, and reduced energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In a glove box with argon atmosphere, weigh 0.911g Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 and 0.089g LiBH 4 (among them, Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 with LiBH 4 The molar ratio is 1:4) into the ball milling tank in turn, the ball-to-material ratio is 120:1, and the ball milling beads are made of stainless steel; the mixture is ball milled in a planetary ball mill at a speed of 300rpm for 12h, and the obtained ball milling product is stored in an argon atmosphere glove box. It was taken out and recorded as LLZTO-LBH, and the subsequent electrochemical performance test was carried out.
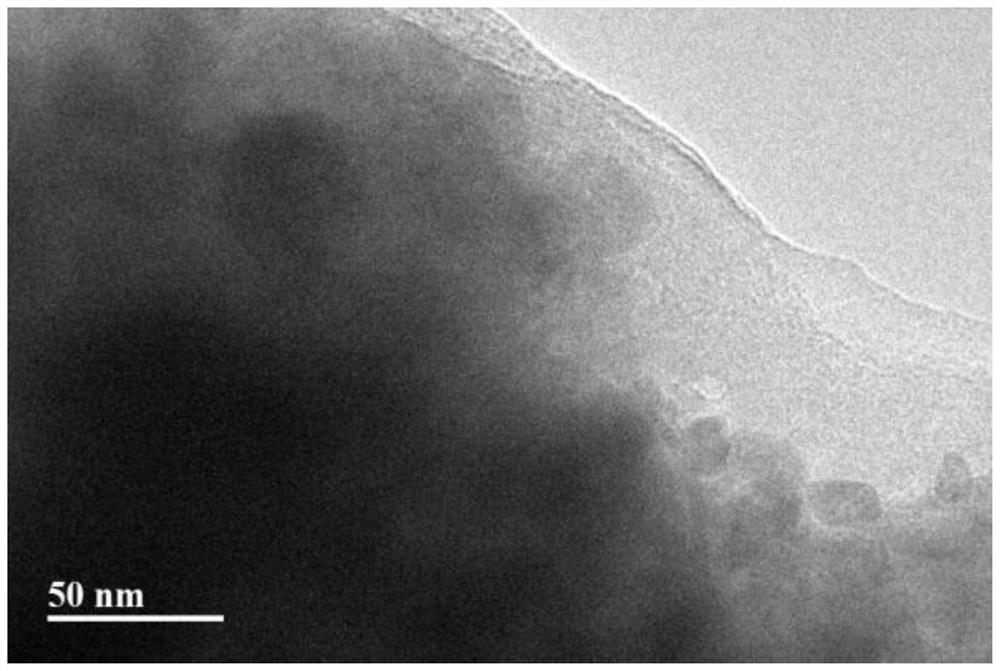
[0040] Such as figure 1 As shown, in the garnet-type composite electrolyte material (LLZTO-LBH) prepared in Example 1, the black and dense garnet-type oxide is covered with a layer of amorphous phase, which improves the compactness of the garnet-type composite electrolyte material. property, reducing the Li + obstacles in the migration process, thereby further improving the Li + ...
Embodiment 2
[0047] In a glove box with argon atmosphere, weigh 0.911g Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 , 0.089g LiBH 4 and 0.053gLiNH 2 (among them, Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 with LiBH 4 、LiNH 2 The molar ratio is 1:4:2) into the ball milling tank in turn, the ball-to-material ratio is 100:1, and the ball milling beads are made of stainless steel; the mixture is ball milled in a planetary ball mill at a speed of 351rpm for 12h, and the obtained ball milling product is heated in an argon atmosphere. Take it out from the glove box, record it as LLZTO-B-N, and conduct subsequent electrochemical performance tests.
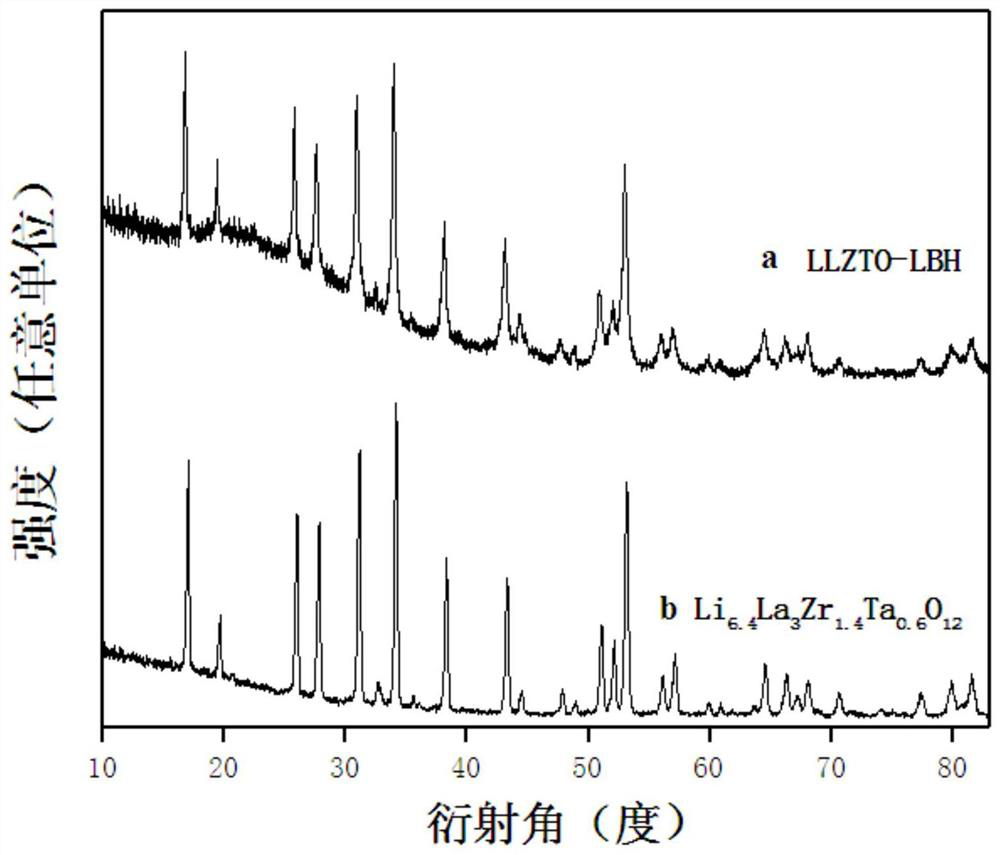
[0048] Such as Figure 7 As shown, A is the XRD spectrum of the garnet-type composite electrolyte material (LLZTO-B-N) prepared in Example 2; B is the original garnet-type oxide (Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 )2's XRD spectrum, as can be seen from the figure, and LiBH 4 The composite material LLZTO-B-N obtained by ball milling has no new crystal phase format...
Embodiment 3
[0051] In the glove box of argon atmosphere, weigh 0.928g Li 6.4 Al 0.2 La 3 Zr 2 o 12 , 0.072g LiBH 4 (among them, Li 6.4 Al 0.2 La 3 Zr 2 o 12 with LiBH 4 The molar ratio is 1:3) and put into the ball milling tank in turn, the ball-to-material ratio is 90:1, and the ball milling beads are made of stainless steel; the mixture is ball milled in a planetary ball mill at a speed of 290rpm for 20h, and the obtained ball milling product is placed in an argon atmosphere glove Take it out of the box, record it as LALZO-LBH, and conduct subsequent electrochemical performance tests.
[0052] Such as Figure 9 Shown, the impedance (EIS) curve of embodiment 3 garnet type composite electrolyte material (LALZO-LBH), the curve is a semicircle in the high-frequency region, and the intersection point of the right end of the semicircle and the real axis represents the total resistance of the bulk phase and the particle gap. The low frequency region is a slanted line, which reflect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com