Mosapride citrate related substances and preparation method thereof
A technology of mosapride citrate and related substances, which is applied in the field of pharmaceutical analytical chemistry, can solve the problems of rare reports and achieve definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 related substance
[0045] Step 1: Take mosapride citrate raw material and citric acid in the same weight ratio and place at 105°C for 17 hours to obtain crude impurities;
[0046] Step 2: After dissolving with 20% acetonitrile aqueous solution, add double water to dilute to prepare a solution with a concentration of 0.03 g / ml, and filter through a 0.45 μm filter membrane to prepare a test solution as an impurity;
[0047] Step 3: the test solution gained in step 2 is entered into a preparative high-performance liquid phase chromatograph for separation; sample size 100ml, flow rate 250ml / min, detection wavelength 274nm, with acetonitrile-0.1% acetic acid aqueous solution (1:1) as mobile phase, Two target peaks were collected, target peak 1 came out at about 14.1min, and target peak 2 came out at about 15.6min. When the two target peaks were detected by the UV detector, start the column to collect fractions and enrich the sample solution ...
Embodiment 2
[0049] The LC-MS detection of embodiment 2 related substance I and related substance II
[0050] 1. Test instrument: WatersAcquity UPLC Q-TOF Prenier MS Thermo LTQ
[0051] 2. Liquid chromatography conditions
[0052] Chromatographic column: silica gel bonded with octadecylsilane (3μm, 150×4.6mm)
[0053] Mobile Phase A: Acetonitrile
[0054] Mobile phase B: 0.1% formic acid in water
[0055] Flow rate: 1ml / min
[0056] Column temperature: 30°C
[0057] Detection wavelength: 274nm
[0058] Gradient elution program:
[0059] time min Flow rateml / min Acetonitrile% 0.1% formic acid in water 0 1.0 15 85 20 1.0 25 75 25 1.0 90 10 35 1.0 90 10 36 1.0 15 85 48 1.0 15 85
[0060] 3. Mass Spectrometry Conditions
[0061] The solution coming out of the UV detector was split, and the flow rate into the mass spectrometer was 0.2ml / min, electrospray ionization, positive ion mode detection, and nitrogen as sheath gas, auxili...
Embodiment 3
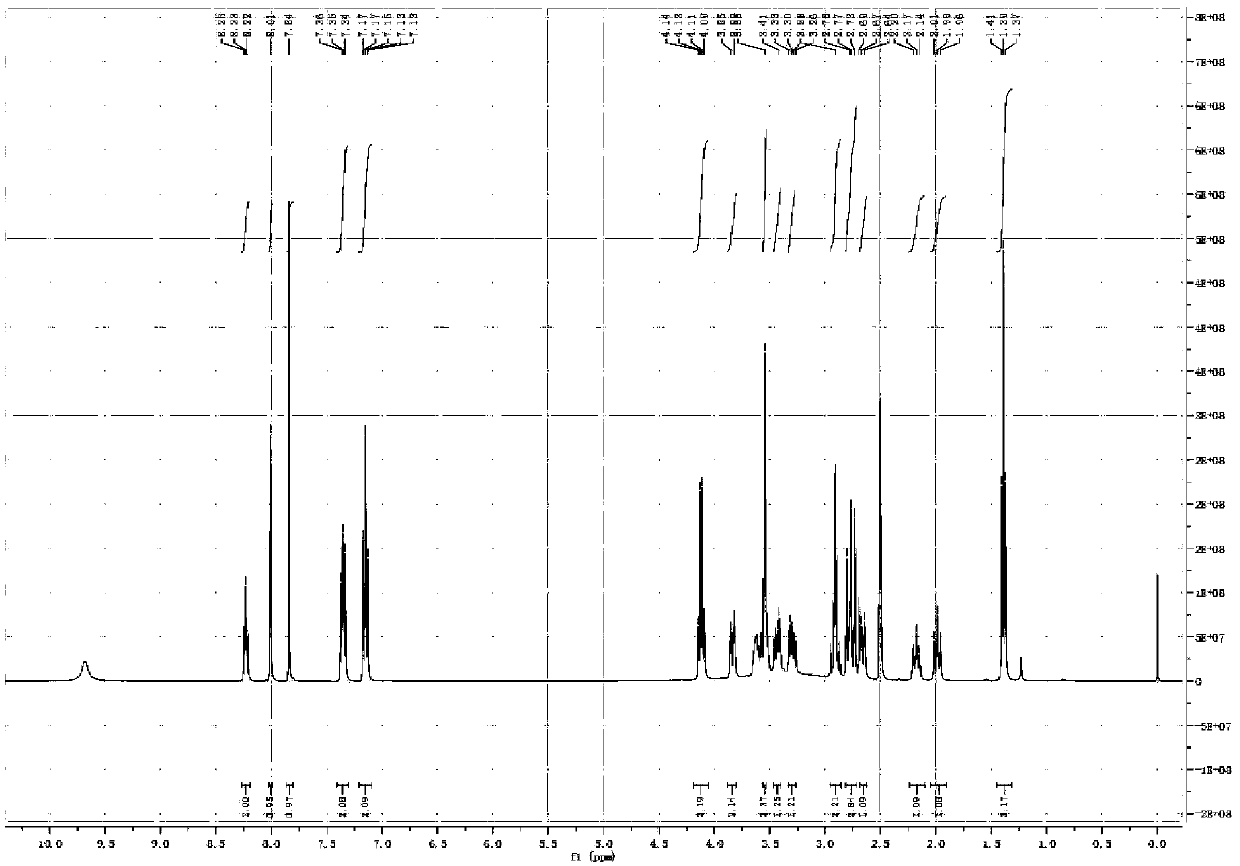
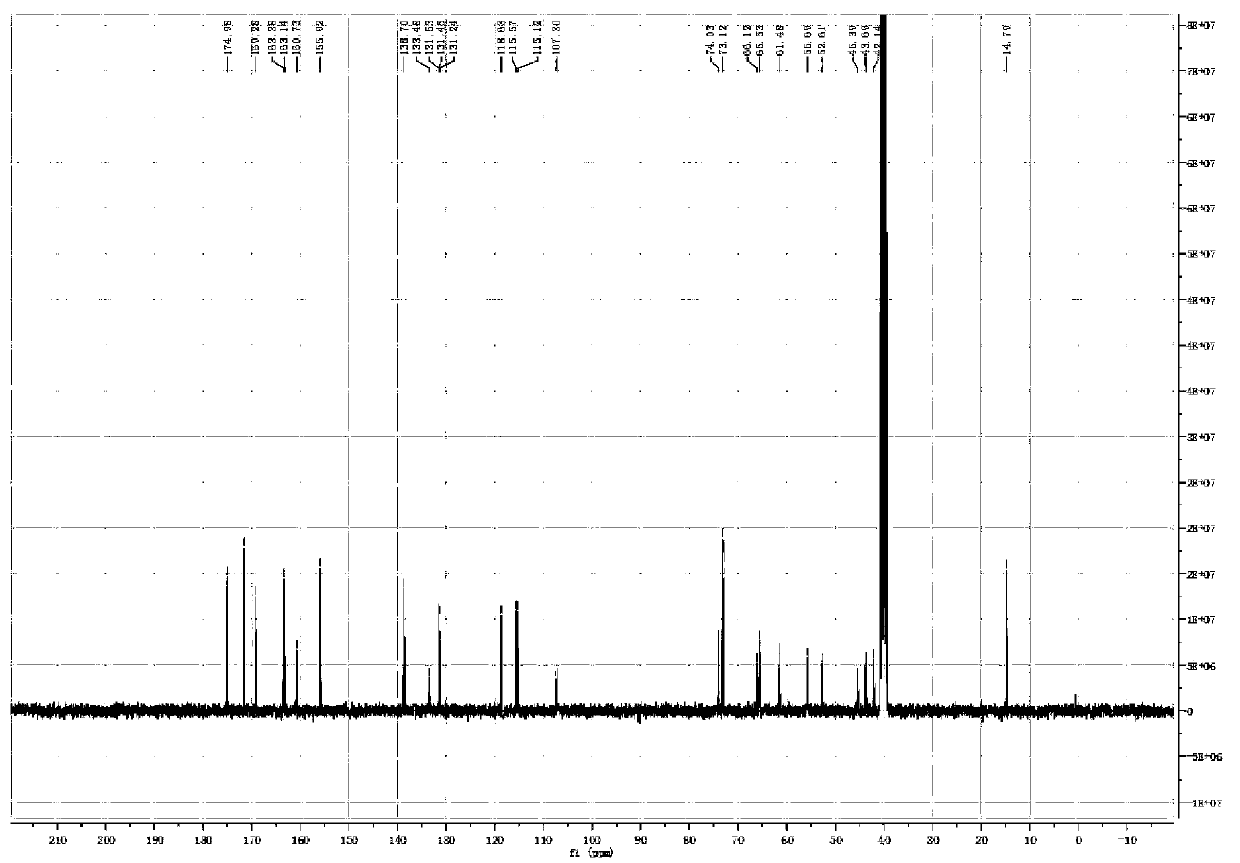
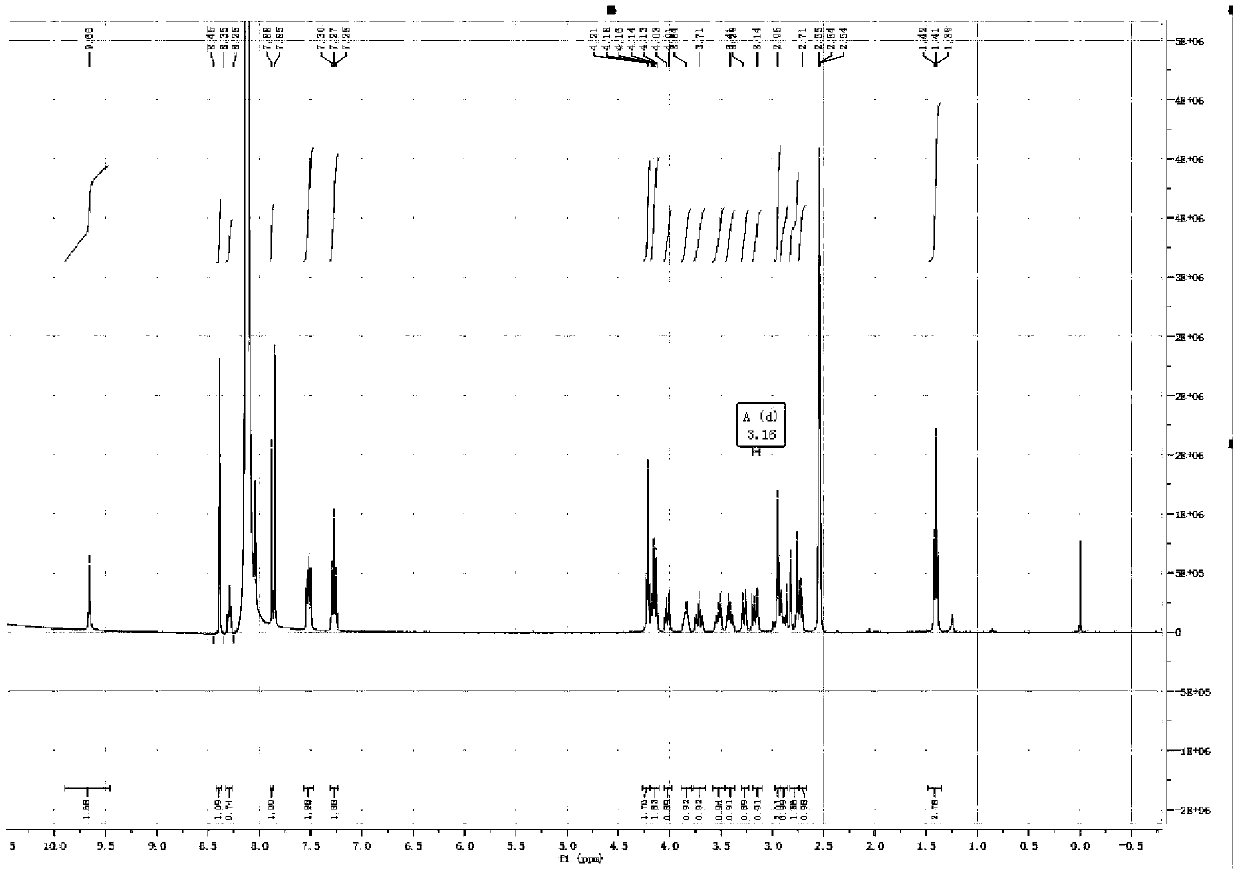
[0065] The proton nuclear magnetic resonance spectrum of embodiment 3 related substance I and related substance II ( 1 H-NMR) spectrum
[0066] 1. Chemical structural formula and hydrogen atom number
[0067]
[0068] 2. Test equipment and conditions
[0069] Test instrument: BrukerAVII-400MHz nuclear magnetic resonance instrument
[0070] Test conditions: TMS internal standard, DMSO as test solvent
[0071] 3. Test results
[0072] For the spectrum of substance I see figure 1 , figure 2 ; For the spectrum of the substance II, see image 3 , Figure 4 .
[0073] The 1H NMR (400MHz, DMSO) of the related substance I was 8.0 (s, 1H), 7.84 (s, 1H), suggesting that it was the hydrogen signal on the benzene ring, and it was 2, 3, 5, 6 four-substituted stupid, 7.35 ( m, 2H), 7.15 (m, 2H) suggest that the compound also has a 1,4 disubstituted benzene ring structure, with a symmetrical structure, 1.4 (t, J = 6.9Hz, 3H), 4.14 (q, J = 6.9Hz , 2H) prompts to contain ethoxy ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com