Preparation method of andrographolide enteric dry suspension
A technology of andrographolide and dry suspension, applied in the field of medicine, can solve problems such as easy degradation of andrographolide, and achieve the effects of improving bioavailability, protecting quality and increasing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The invention discloses a preparation method of andrographolide enteric-coated dry suspension, comprising the following steps:
[0042] (1) Preparation of particulate matter
[0043] Dissolve hydroxypropyl cellulose with 20% to 95% ethanol aqueous solution and add sodium lauryl sulfate to prepare a solvent with a solid content of 3% to 5% as a binder;
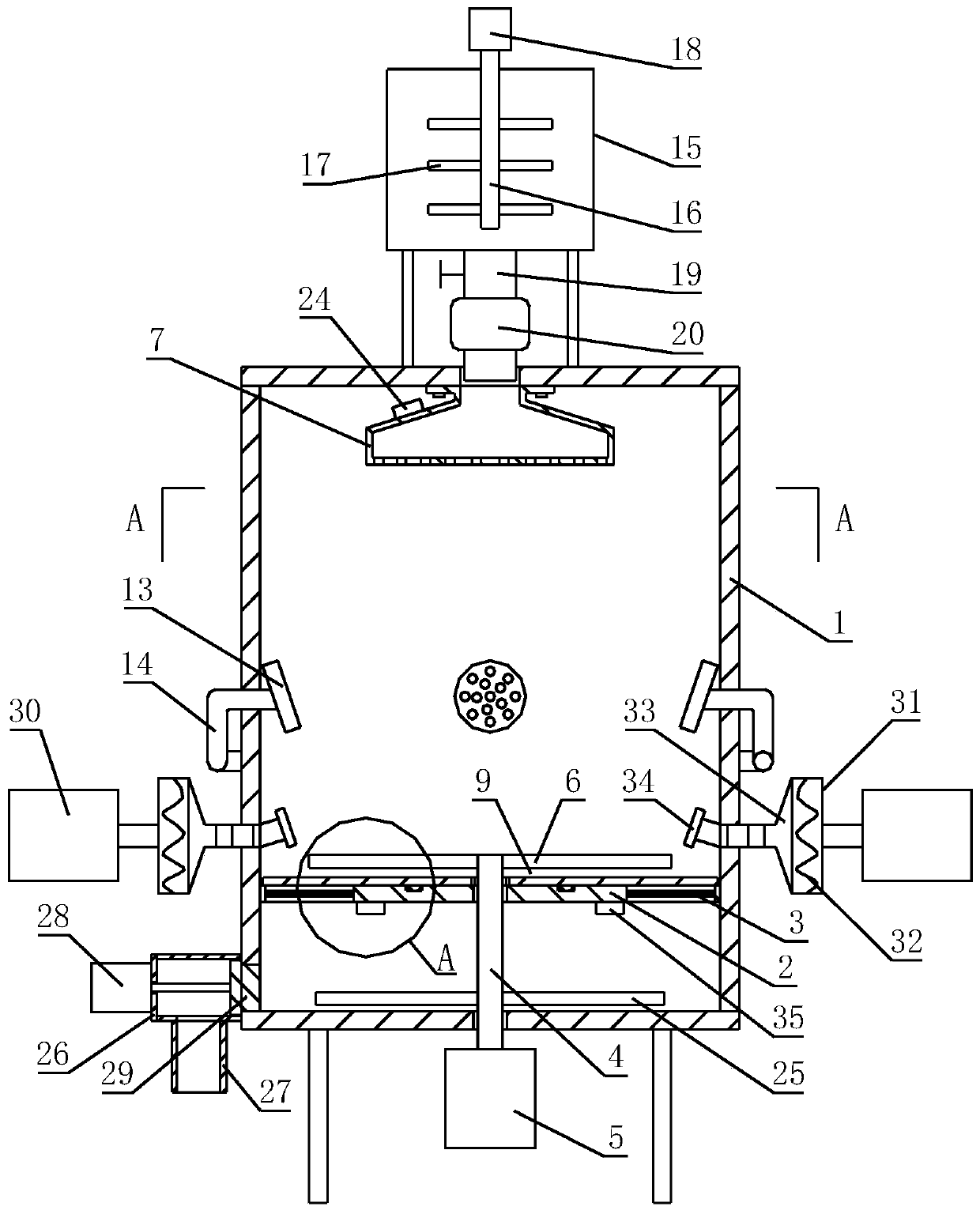
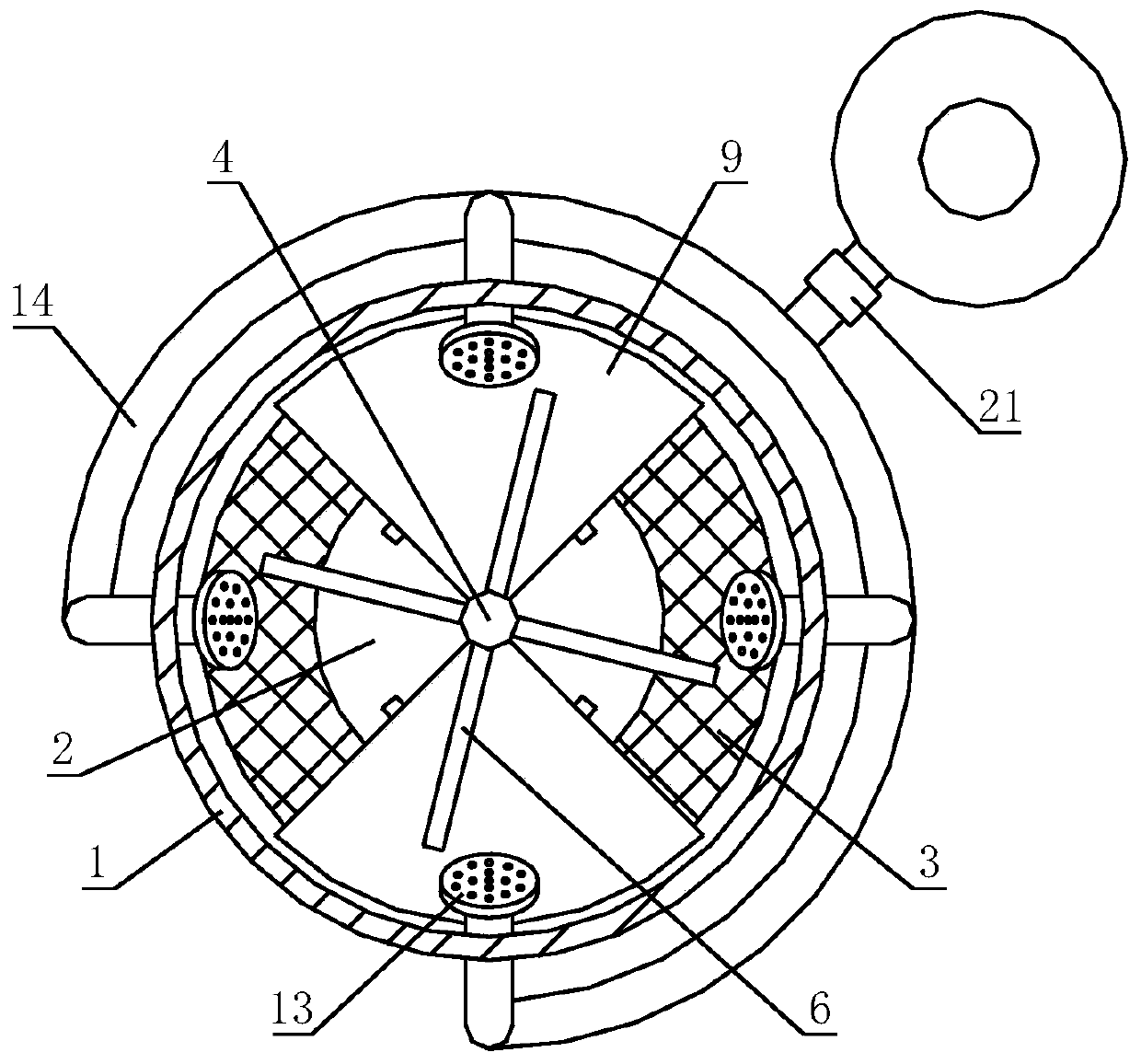
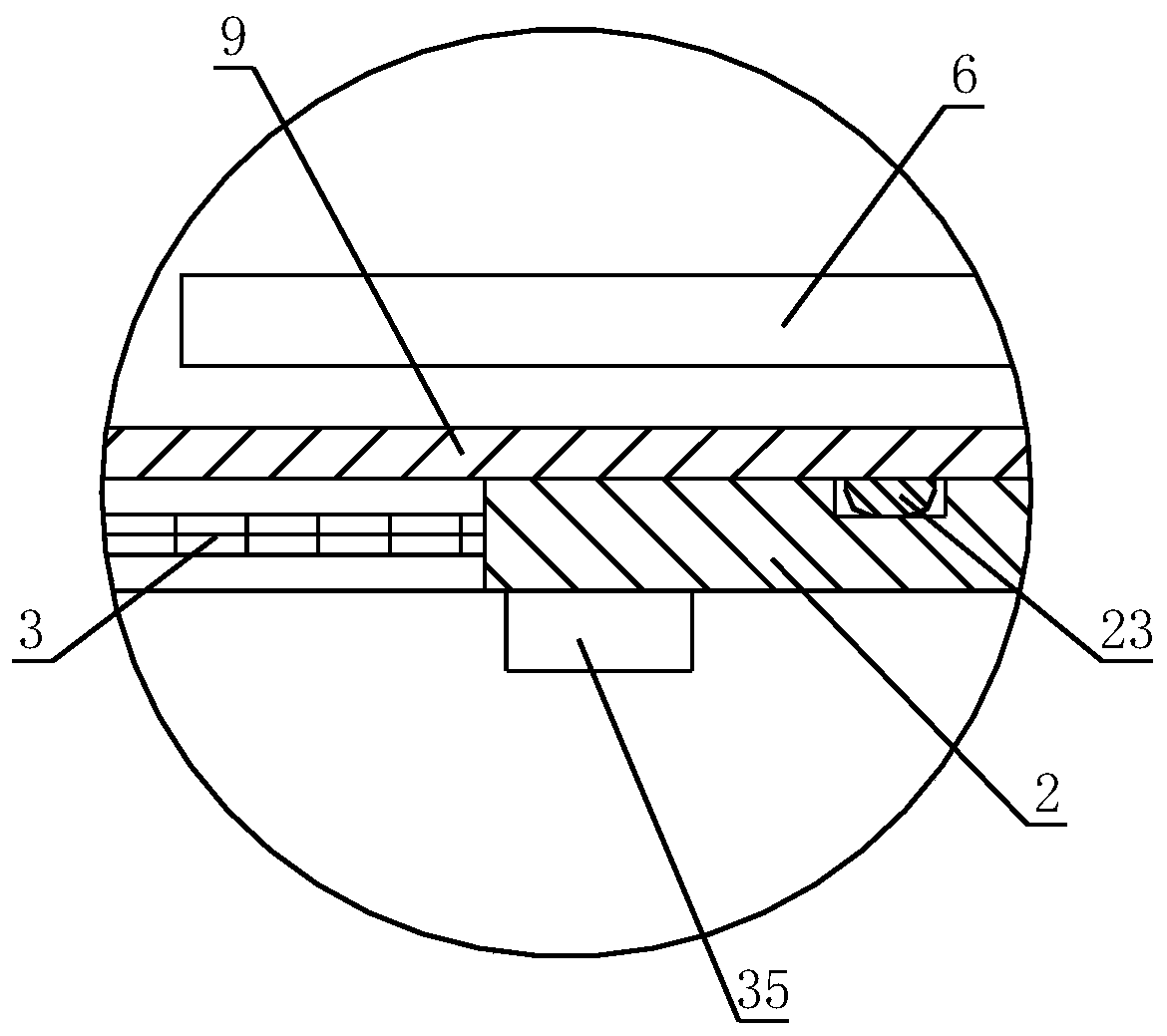
[0044] After sieving andrographolide, lactose and microcrystalline cellulose, add them to a high-speed centrifugal granulation coating machine, and carry out spray granulation with the binder. The mesh number of andrographolide, lactose and microcrystalline cellulose sieved In practice, conventional settings are required; dry the granules after they are formed; the spray rate of the high-speed centrifugal granulator coating machine is 2-15mL / min, the speed is 100-300r / min, and the rotation is 10-30min; the drying temperature is 40°C, and the drying time is 8-12 hours, the dried particles pass through a 60-100 mesh sieve...
Embodiment
[0067] The above method was used to prepare andrographolide enteric-coated dry suspension, and the specific composition ratios are shown in Table 1 and Table 2 below.
[0068] Table 1 Raw material ratio (parts by weight)
[0069]
[0070] Table 2 Raw material ratio (parts by weight)
[0071]
[0072]
[0073] Andrographolide enteric-coated dry suspension was prepared according to the ratios in Table 1 and Table 2, and the process parameters for preparing granules by a high-speed centrifugal granulator coating machine are shown in Table 3 below.
[0074] Table 3 Process parameters for the preparation of particulate matter
[0075] group Speed / r·min -1
Rotation time / min Spray rate / mL2min -1
Drying time / h 1 160 20 8 10
[0076] After the preparation of the granules is completed, the granules are coated with an isolation layer and an enteric layer, and the process parameters of the coating machine during the process of coating the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com