Preparation method for catalytic synthesis of 4,4'-dichlorophenylsulfone
A dichlorophenyl sulfone and chemical synthesis technology, applied in the field of compound preparation, can solve the problems of complex process, low product melting point, low conversion rate, etc., and achieve the effects of high product purity, good economic benefits, and low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
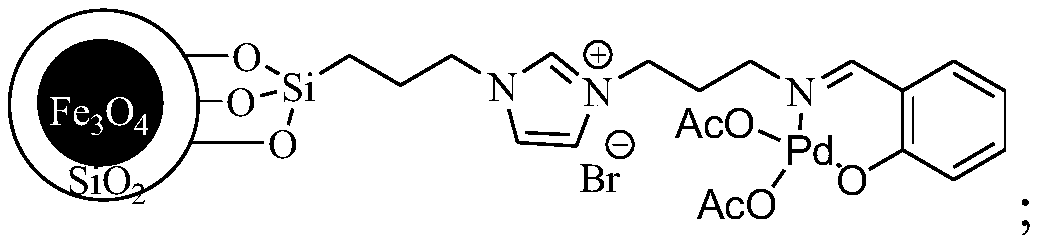
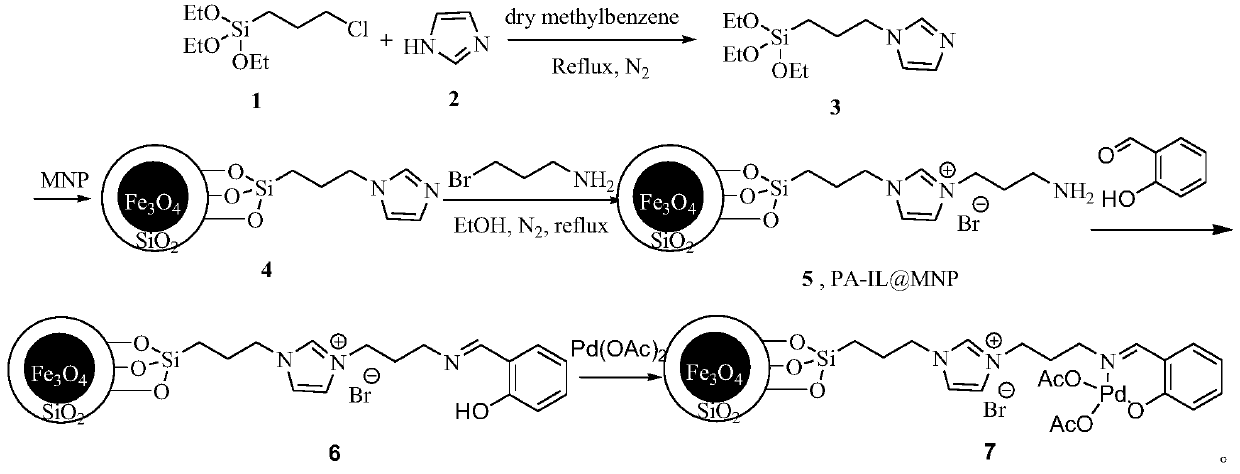
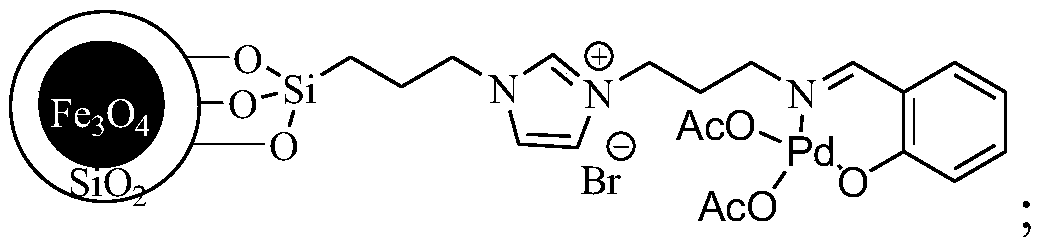
[0016] A preparation method for catalyzing and synthesizing 4,4'-dichlorophenyl sulfone, the preparation process of the superparamagnetic nanoparticle-loaded ionic liquid catalyst used in the present invention:
[0017] Weigh respectively 3-chloropropyltriethoxysilane 1 (12ml, 50mmol), imidazole 2 (3.4g, 50mmol) in a reaction vessel, add 50ml of dry toluene, N 2 Under protection, reflux and stir the reaction for 24h, and obtain intermediate 3 through column chromatography (developing agent is EA); take 1g Fe 3 o 4 / SiO 2 Put the solid particles in the reaction vessel, add 50ml of anhydrous toluene, and ultrasonicate for 1h. After the ultrasonication is over, take 0.5g of intermediate 3 and dissolve it in 20ml of anhydrous toluene, then add it dropwise to the reaction system, N 2 Reflux and stir the reaction for 48 hours under protection. After the reaction, collect it with a magnet and wash it three times with ethanol, and dry it in a vacuum to obtain the solid particle 4; t...
Embodiment 1
[0024] In the four-necked flask, add chlorobenzene (1.0mol, 112.5g) first, then add the catalyst (0.34g, 0.2mmol), stir well and add thionyl chloride (0.2mol, 23.8g) dropwise, the reaction exothermic, control The temperature is 30°C. After adding the thionyl chloride dropwise, keep stirring for 1 h. After the reaction is over, add the catalyst recovered by the magnetic field, pour out the reaction solution, add a saturated sodium bicarbonate solution dropwise to the reaction solution to adjust the pH value to neutral, and stir for 0.5 h. Filtrate while it is hot, cool the filtrate to 0-5°C for crystallization, filter and dry to obtain solid 4,4'-dichlorodiphenylsulfoxide (0.19mol, 51.2g), heat and dissolve with glacial acetic acid (0.3mol, 18g), 60 Add 35% hydrogen peroxide (0.3mol, 30g) dropwise at ℃ for oxidation, keep warm for 2h, cool down, filter and dry to obtain 4,4'-dichlorodiphenylsulfone (0.18mol, 51.8g), the content is 99.5% (HPLC detection), The yield was 90.5%. ...
Embodiment 2
[0026] First add chlorobenzene (1.2mol, 135g) in the four-necked flask, then add the catalyst (0.34g, 0.2mmol), stir well and add thionyl chloride (0.2mol, 23.8g) dropwise, the reaction exothermic, control the temperature 30°C. After adding the thionyl chloride dropwise, keep stirring for 1 h. After the reaction is over, add the catalyst recovered by the magnetic field, pour out the reaction solution, add a saturated sodium bicarbonate solution dropwise to the reaction solution to adjust the pH value to neutral, and stir for 0.5 h. Filtrate while it is hot, cool the filtrate to 0-5°C to crystallize, filter and dry to obtain solid 4,4'-dichlorodiphenylsulfoxide (0.185mol, 50.0g), heat and dissolve with glacial acetic acid (0.3mol, 18g), 60 Add 35% hydrogen peroxide (0.3mol, 30g) dropwise at ℃ for oxidation, keep warm for 2h, cool down, filter and dry to obtain 4,4'-dichlorodiphenylsulfone (0.17mol, 50.0g), the content is 99.6% (HPLC detection), The yield was 87.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com