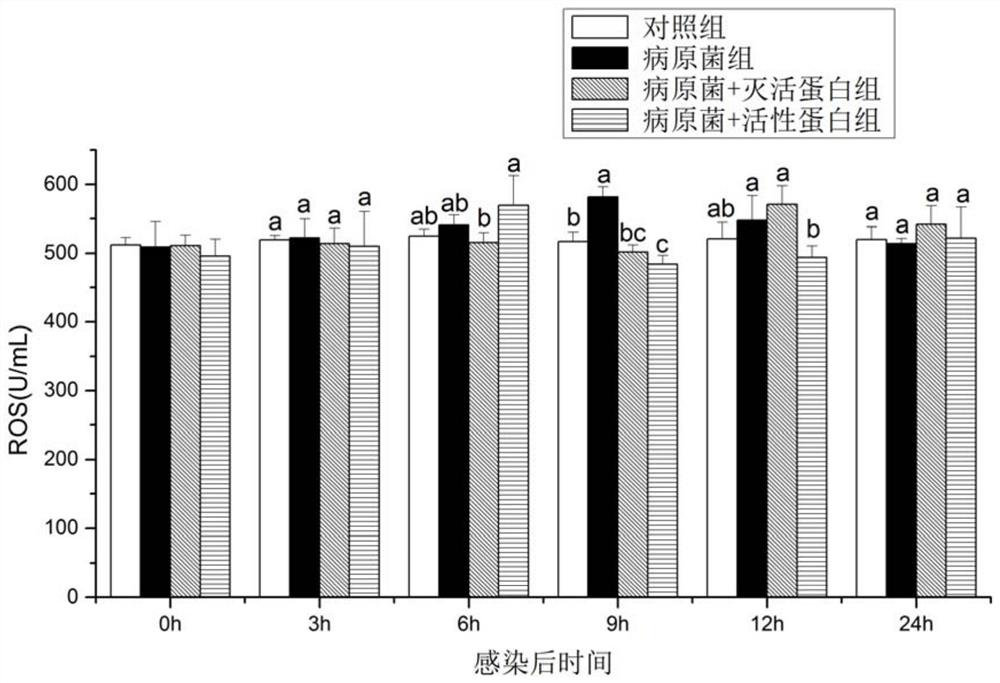
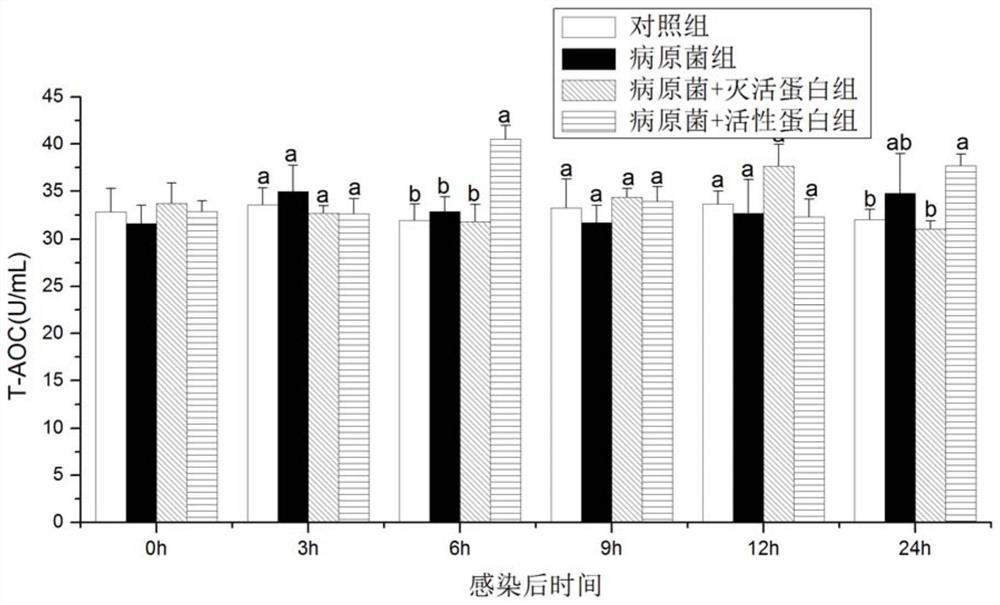
A kind of preparation method and application of recombinant protein swho1
A recombinant protein, HO1 technology, applied in the field of preparation of recombinant protein swHO1, to achieve the effect of improving serum total antioxidant capacity, increasing expression, and balancing oxidative stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Cloning of the eel HO1 gene
[0021] (1) Extract the total RNA of the eel liver according to the Trizol reagent instructions, use M-MLV reverse transcriptase to synthesize cDNA, and store the synthesized cDNA at -80°C for later use;
[0022] (2) According to the known HO1 gene sequence of fish, a pair of degenerate primers de-HO1-F (as shown in SEQ ID NO: 1, 5'-CACRGARCTBATGCTGAGCT-3') and de-HO1-R ( As shown in SEQ ID NO: 2, 5'-CTGSTGGCCCACGCKTACACC-3') is used to amplify the cDNA fragment of the eel HO1 gene obtained by the above-mentioned reverse transcription synthesis;
[0023] (3) After the amplification product is purified, it is connected to the pEASY-T1 vector, transformed into DH5a competent cells, and identified as a positive clone fragment;
[0024] (4) Gene-specific primers HO5GSP (as shown in SEQ ID NO: 3, 5'-ATCTACCAGGCCCTGGAGGAAGAGATGGACAGGAAC-3') and HO3GSP (as shown in SEQ ID NO: 4, 5'-GGAGGTCAGGTCTTGGGTCGAATCGCTC-3') were designed accor...
Embodiment 2
[0026] Example 2 Construction of prokaryotic expression vector
[0027] According to the Trizol kit instructions, the total RNA from the liver tissue of the eel was extracted and the first strand of cDNA was synthesized by MMLV reverse transcriptase.
[0028] Using the full-length cDNA sequence of the eel HO1 gene prepared in Example 1 as a template, and re-ex-F and re-ex-R as primers, PCR amplification was performed. The PCR product and pET-28a(+) expression vector were double digested, recovered and purified, ligated and transformed into Escherichia coli DH5α, and the recombinant expression vector pET-HO1 was obtained.
[0029] Specifically, specific primers re-ex-F (as shown in SEQ ID NO: 6, 5'-CAAGGATCCATGGAAGCAGAGAAGAAAAC-3') and re-ex-R (as shown in SEQ ID NO: 7, 5'-CAA CTCGAG TAAAACGTAGATTCCCATA-3'), BamHI and XhoI restriction sites were added to the forward primer re-ex-F and reverse primer re-ex-R, respectively.
[0030] Using primers re-ex-F and re-ex-R to ampli...
Embodiment 3
[0032] Example 3 Induction expression and purification of recombinant protein pET-HO1
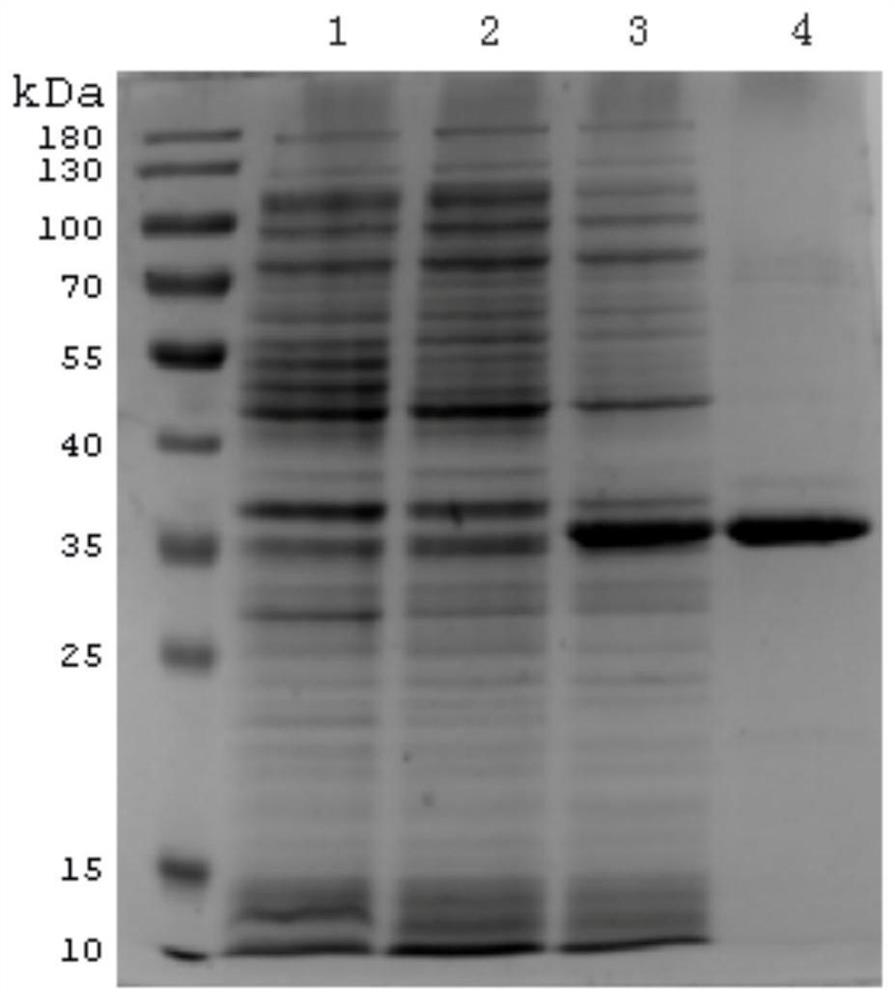
[0033] 1 ng of the recombinant expression vector pET-HO1 verified by sequencing in the above Example 1 was transformed into E.coli BL21 (DE3) bacteria, and cultured in LB medium containing 50 mg / L kanamycin to an OD value of about 0.6, then added IPTG (125μg·mL -1 ) were induced and cultured for 3 h. The bacterial cells were collected by centrifugation, sonicated, and then added with 5× loading buffer for 12% SDS-PAGE electrophoresis to detect the expression of the target protein.
[0034] The BL21(DE3) bacterial strain capable of expressing recombinant protein was inoculated into fresh LB medium containing 50 μg / mL kanamycin with 1% inoculation amount (mass volume ratio), and cultured at 200r / min at 37°C for 12-15h, Induced expression. Centrifuge the induced bacterial solution, collect the bacterial cells by centrifugation at 4°C, 10000 r·min-1 for 10 min, resuspend the pellet with PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com