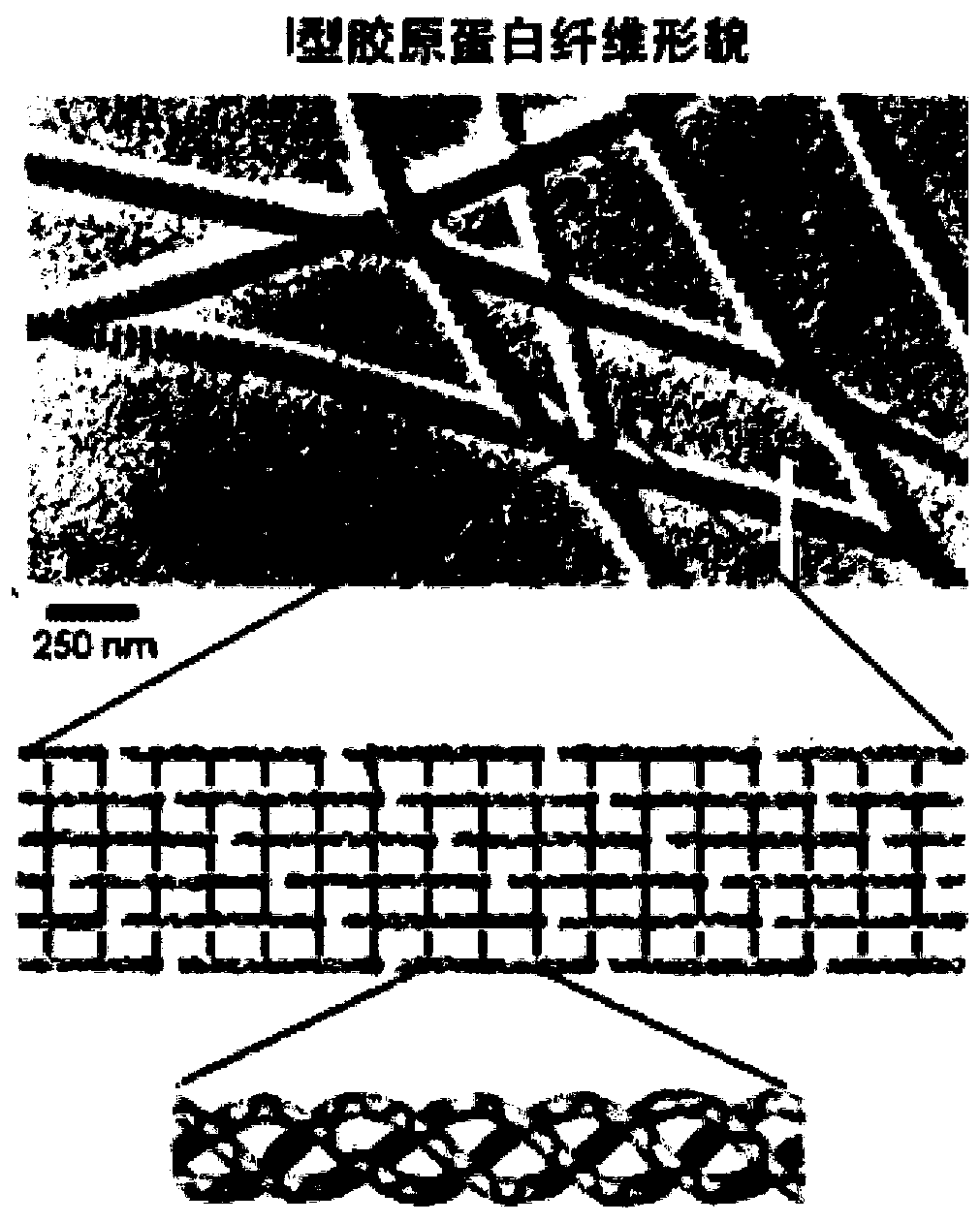
Preparation method of type I collagen-like fiber
A collagen fiber and collagen technology, applied in the field of genetic engineering, can solve the problems of limited application, inability to form collagen fibers, and inability to form nanofiber morphology, etc., and achieve the effect of simple preparation process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Sequence Design and Collagen Preparation
[0059] according to The structure shown is designed, and the specific steps are:
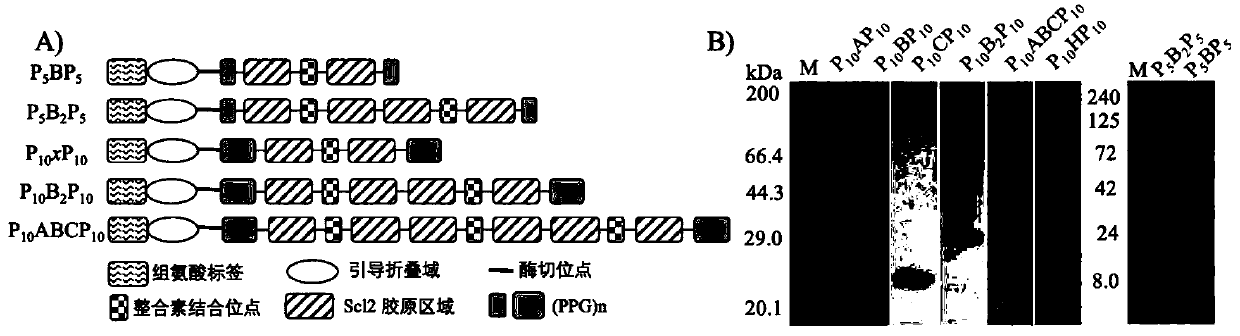
[0060] (1) With N and C terminals (GPP) 10 As a fixed sequence motif, a variable collagen region is inserted in the middle to obtain a three-segment chimeric sequence (abbreviated as P 10 CLP 10 ). In this example, the CL-domain adopts the amino acid sequence (abbreviation For H) as bacterial collagen, wherein the Scl2 collagen region is divided into three equal-length regions A, B and C, in the following examples, the designed CL domains are respectively A, B, C, and BB (repeated 2 a B region) and ABC (equivalent to the complete Scl2 collagen region).
[0061] (2) A globular domain derived from Scl2 (shown in SEQ ID NO.1) is inserted at the N-terminus of the sequence to guide the correct folding of the collagen triple helix, and a protease cleavage site is inserted between the globular domain and the fixed sequence unit of the...
Embodiment 2
[0078] Example 2 Determination of the secondary structure of collagen
[0079] The collagen prepared in Example 1 was made into a concentration of 1 mg / mL. Then stand at 4°C for more than 24h, use a 1mm cuvette, scan the full wavelength of circular dichroism at 4°C, the wavelength is from 190nm to 260nm, the wavelength interval is 1nm, and stay at each wavelength for 5s. The thermal change experiment was measured at 220nm, the temperature was from 4°C to 80°C, each temperature was equilibrated for 8s, and the temperature increment rate was 1°C / 6min. The typical CD spectrum of collagen triple helix structure shows a positive absorption peak at 220nm.
[0080] like Figure 4 As shown, under full-wavelength scanning, the protein designed in Example 1 has a characteristic absorption peak around 220nm; the thermal variation experiment results show that as the temperature increases, the characteristic absorption value at 220nm changes suddenly at around 50°C, It is manifested by ...
Embodiment 3
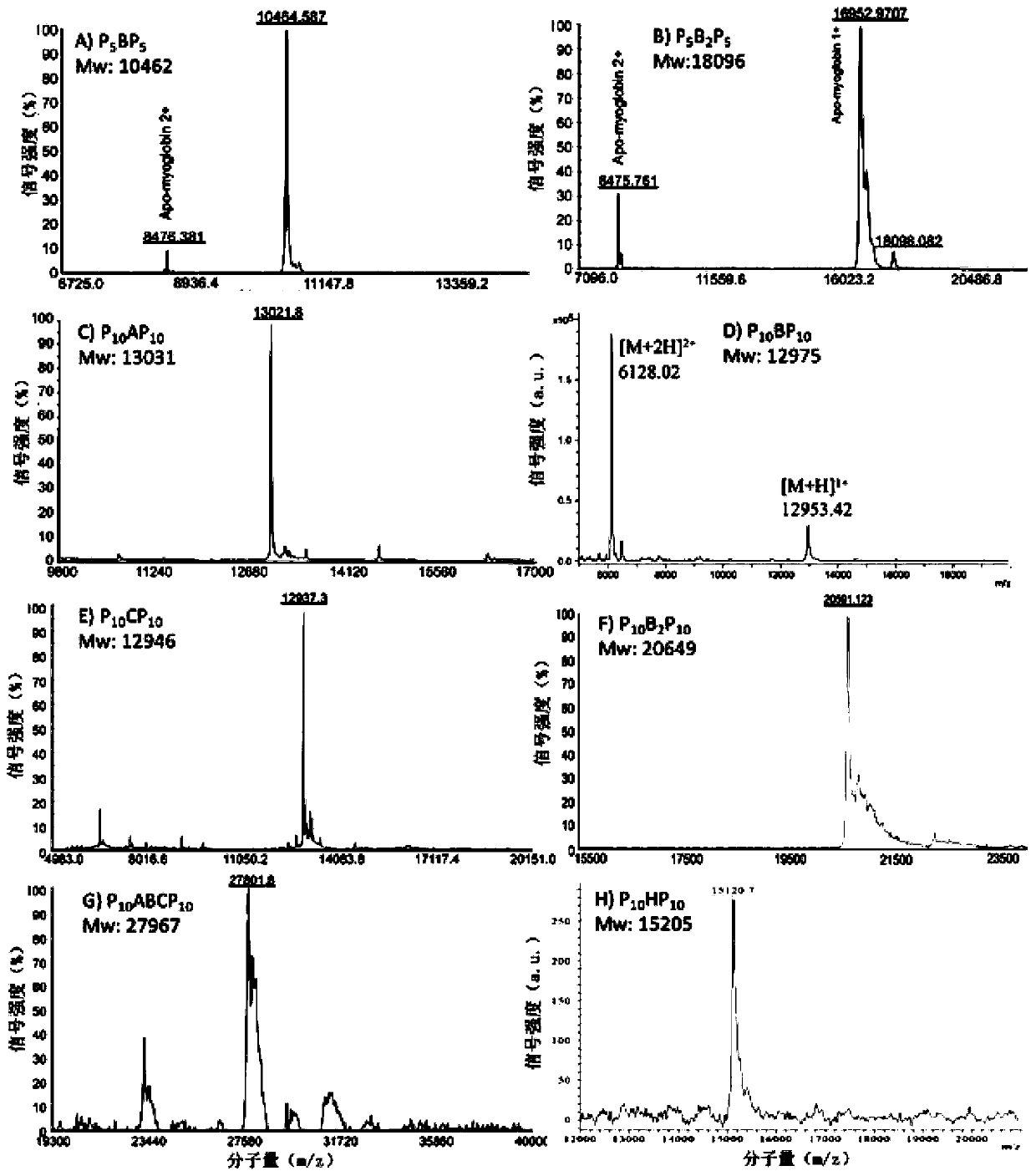
[0081] Example 3 Effects of Substitution of Collagen Domain Sequence on Fiber Structure
[0082] The lyophilized collagen P prepared in Example 1 10 AP 10 ,P 10 BP 10 ,P 10 CP 10 , P 10 HP 10 Use 10mM PB to prepare a solution with a final concentration of 0.5mM, place it at 4°C for 3.5 days, take a small amount and drop it on the copper grid, absorb it for 45s, then blot it dry with filter paper, then negatively stain it with 0.75% phosphotungstic acid for 20s, and blot it dry on the filter paper , observed with a Hitachi H-7650 transmission electron microscope, as Figure 5 According to the transmission electron microscopy results shown, the designed collagen can self-assemble to form ribbon fibers with periodic light and dark stripes, and the periodic light and dark stripes formed by sequences A, B and C have the same length. by negatively staining P 10 BP 10 The light and dark lines of the fiber are measured, and the lengths of the light and dark lines are respect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com