Synthesis method of trans-4-alkyl cyclohexyl benzoic acid
A technology of cyclohexyl benzoic acid and cyclohexyl benzoate, which is applied in the field of organic chemical synthesis, can solve the problems of cyclohexyl benzene being difficult to obtain, large environmental pollution, etc., and achieves easy industrial production, little environmental pollution, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
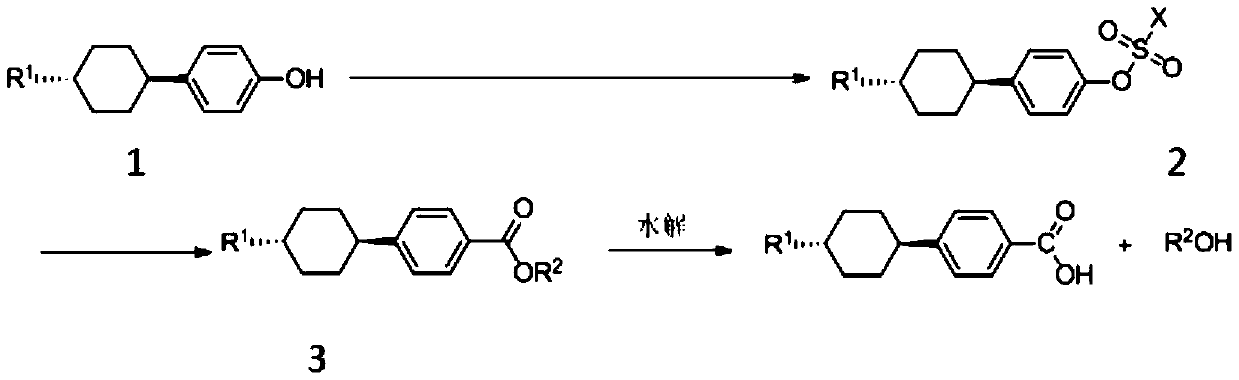
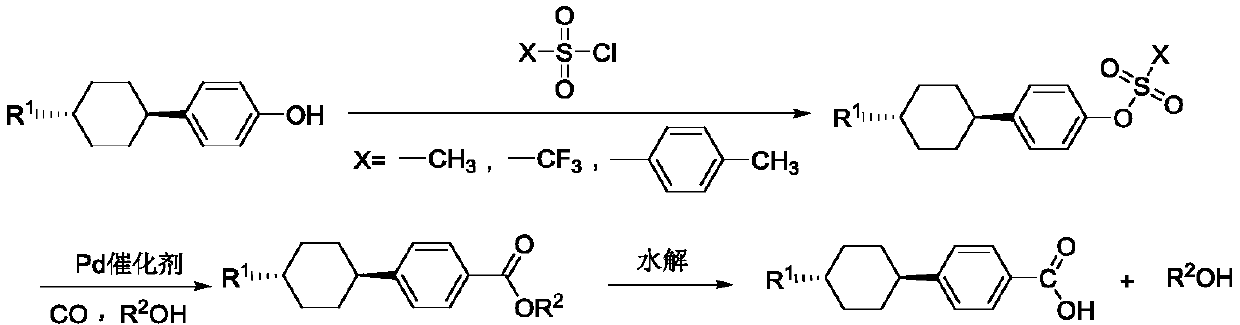
[0032] Add trans-4-methylcyclohexylphenol (0.8mol, 153.23g), sodium carbonate (0.8mol, 84.79g), toluene (500mL) into a 2000mL three-necked flask, stir for 15min, reflux for 2 hours to remove the water in the system , The temperature was lowered to 80° C., p-toluenesulfonyl chloride (1.0 mol, 190.65 g) dissolved in anhydrous toluene was slowly added dropwise, and after the dropwise addition, the temperature was raised and refluxed for 10 hours. After the reaction, it was cooled, washed with water until the organic phase was neutral, the water phase was separated, the organic phase was distilled under reduced pressure to recover the solvent, and the remaining was cooled and dried to obtain trans-4-methylcyclohexylbenzene p-toluenesulfonate (268.71g) ), the content is 91% detected by liquid chromatography;
[0033] The prepared trans-4-methylcyclohexylbenzene p-toluenesulfonate (0.1mol, 37.86g), isopropanol (0.3mol, 18.02g), palladium dichloride (0.001mol, 0.18g), Tripheny...
Embodiment 2
[0036]
[0037] Add trans-4-ethylcyclohexylphenol (0.8mol, 163.45g), potassium carbonate (0.8mol, 110.57g), toluene (500mL) into a 2000mL three-necked flask, stir for 15min, reflux for 2 hours to remove the water in the system After the temperature was lowered to 80°C, methylsulfonyl chloride (1.0 mol, 114.56 g) was slowly added dropwise, and after the dropwise addition, the temperature was raised and refluxed for 10 hours. After the reaction, cool and wash with water until the organic phase is neutral. Separate the water phase and distill the organic phase under reduced pressure to recover the solvent. After cooling and drying, the remaining white substance is trans-4-ethylcyclohexylbenzyl sulfonic acid. Ester (233.05g), the content is 92% detected by liquid chromatography;
[0038] The prepared trans-4-ethylcyclohexylbenzenesulfonate (0.1mol, 30.67g), n-butanol (0.3mol, 22.24g), palladium acetate (0.002mol, 0.45g), bis(diphenyl) Phosphine) methane (0.002mol, 0.77g), potassium ...
Embodiment 3
[0041]
[0042] Add trans-4-propylcyclohexylphenol (0.8mol, 174.67g), triethylamine (0.8mol, 81.0g), toluene (500mL) into a 2000mL three-necked flask, stir for 15min, reflux for 2 hours to remove the With water, the temperature was lowered to 80°C and trifluoromethanesulfonyl chloride (1.0 mol, 168.52g) was slowly added dropwise. After the dropwise addition, the temperature was raised and refluxed for 10 hours. After the reaction, cool, wash with water until the organic phase is neutral, separate the water phase, and distill the organic phase under reduced pressure to recover the solvent. After cooling and drying, the remaining white substance is trans-4-propylcyclohexyltrifluoromethanesulfonic acid Ester (276.53g), the content is 93% detected by liquid chromatography;
[0043] The prepared trans-4-propylcyclohexyl trifluoromethanesulfonate (0.1mol, 36.36g), ethanol (200mL), bisacetonitrile palladium dichloride (0.001mol, 0.26g), two (diphenyl) Phosphine) ethane (0.001mol, 0.4g)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com