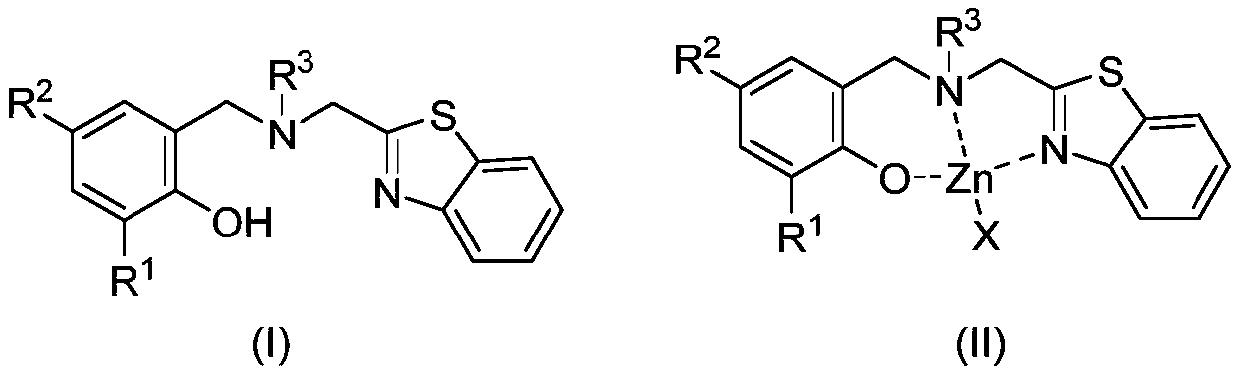
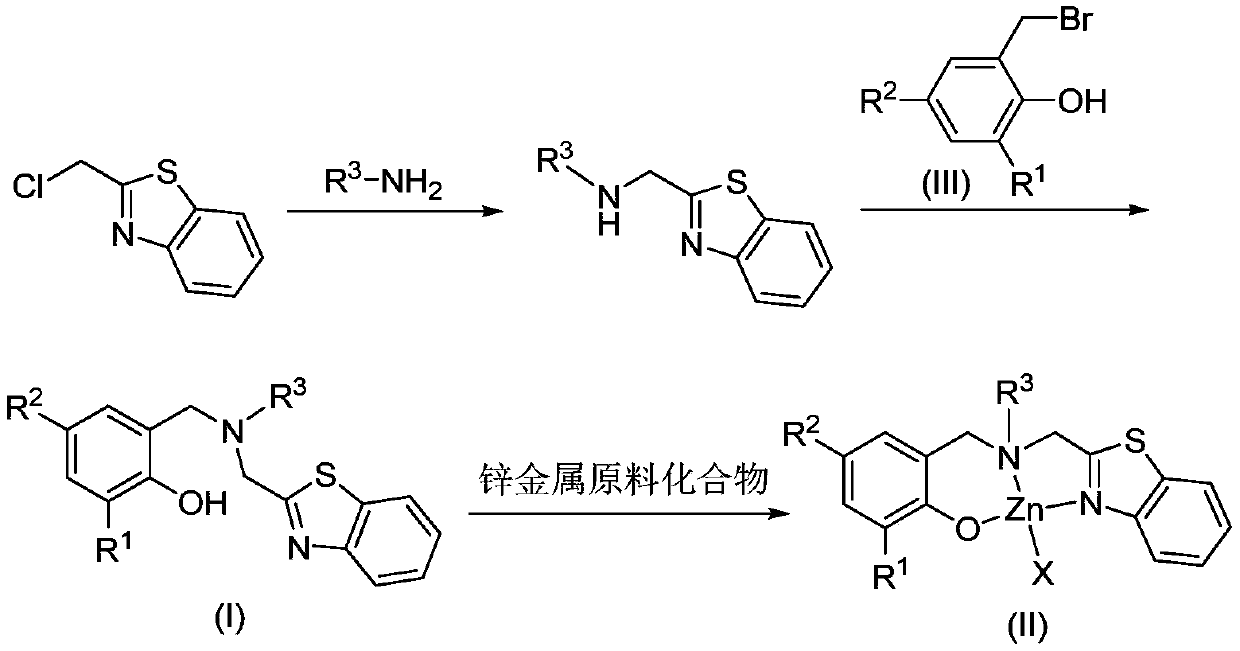
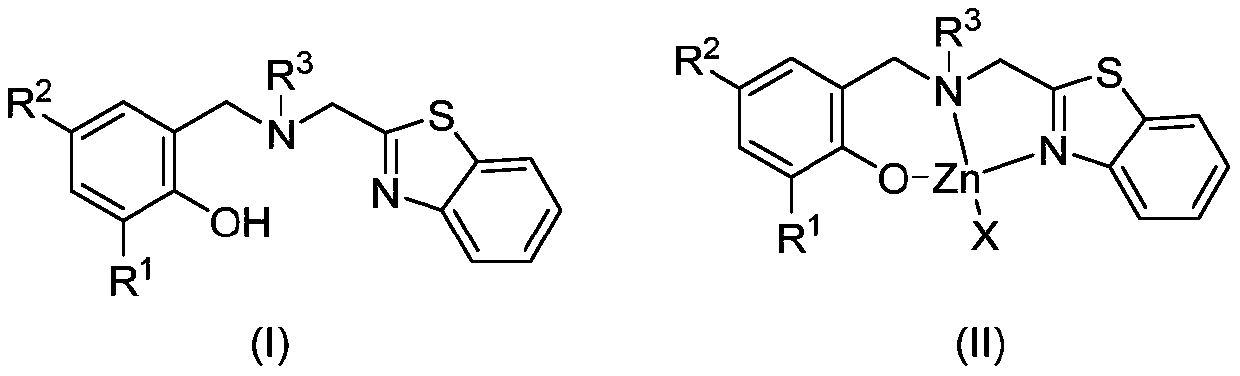
Benzothiazole ring substituted aminophenoxy zinc complex, and preparation method and application thereof
A technology of aminophenoloxyzinc and benzothiazole rings, which is applied in the field of aminophenoloxyzinc complexes, can solve the problems of catalyst sensitivity, no stereoselectivity, and low catalyst activity, and achieve stable properties and high isotactic stereo Effect of selectivity and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Ligand L1:
[0044] (1) Synthesis of N-[(benzothiazol-2-yl)-methyl]cyclohexylamine
[0045]
[0046] Under the protection of inert gas, add cyclohexylamine (24.5mmol, 2.43g) and anhydrous K 2 CO 3 (2.94mmol, 0.41g), was added dropwise a solution of 2-chloromethylbenzothiazole (2.45mmol, 0.45g) in 50mL of N,N-dimethylformamide from a constant pressure dropping funnel, and reacted for 8h. Quenched with water, extracted with dichloromethane, washed with saturated brine and washed with anhydrous MgSO 4 Dry, filter, and distill off the solvent under reduced pressure to obtain a yellow viscous liquid, and distill off unreacted cyclohexylamine at 90°C / 1mmHg. The main product point was analyzed by TLC, and it was directly used in the next reaction, and the yield was about 80% according to the H NMR spectrum.
[0047] (2) Synthesis of Ligand L1
[0048] Add N-[(benzothiazol-2-yl)-methyl]cyclohexylamine (5mmol, 1.23g), anhydrous potassium carbonate (5.5mmol, ...
Embodiment 2
[0052] Synthesis of Ligand L2
[0053] (1) Synthesis of N-[(benzothiazol-2-yl)-methyl]benzylamine
[0054]
[0055] Except that benzylamine (16.07g, 150mmol), potassium carbonate (2.28g, 16.5mmol) and 2-chloromethylbenzothiazole (2.75g, 15mmol) were used as raw materials, other operating steps were the same as in Example 1. An orange-red oil was obtained.
[0056] (2) Synthesis of Ligand L2
[0057] N-[(benzothiazol-2-yl)-methyl]benzylamine (14mmol, 3.56g), anhydrous potassium carbonate (15.4mmol, 2.13g) and 2-bromomethyl-4-methyl -Except for 6-tritylphenol (14mmol, 6.34g), other operations were the same as in Example 1 (5.50g, 64%).
[0058]
[0059] 1 H NMR (400MHz, CDCl 3 ,298K):δ9.49(s,1H,OH),7.97(d, 3 J=8.1Hz,1H,ArH),7.85(d, 3 J=7.9Hz,1H,ArH),7.54–7.44(m,1H,ArH),7.40(m, 3 J=11.2Hz,1H,ArH),7.29–7.17(m,15H,ArH),7.13(t, 3 J=6.8Hz, 3H, ArH), 7.04(m, 3 J=14.0,10.5Hz,2H,ArH),6.92(s,1H,ArH),6.84(s,1H,ArH),3.89(s,2H,ArCH 2 ),3.86(s,2H,NCH 2 C=N),3.60(s,2H,PhCH ...
Embodiment 3
[0061] Synthesis of Ligand L3
[0062] (1) Synthesis of N-[(benzothiazol-2-yl)-methyl]n-hexylamine
[0063]
[0064] Except that n-hexylamine (15.18g, 150mmol), potassium carbonate (2.28g, 16.5mmol) and 2-chloromethylbenzothiazole (2.75g, 15mmol) were used as raw materials, other operating steps were the same as in Example 1. An orange-red oil was obtained.
[0065] (2) Synthesis of Ligand L3
[0066] In addition to raw materials, N-[(benzothiazol-2-yl)-methyl]n-hexylamine (13.85mmol, 3.44g), anhydrous potassium carbonate (16mmol, 2.21g) and 2-bromomethyl-4-methyl - Except for 6-tritylphenol (13.85mmol, 6.14g), other operations were the same as in Example 1 (6.30g, 73%).
[0067]
[0068] 1 H NMR (400MHz, CDCl 3 ,298K):δ9.45(s,1H,OH),7.96(d, 3 J=7.9Hz,1H,ArH),7.84(d, 3 J=7.6Hz,1H,ArH),7.51–7.43(m,1H,ArH),7.40(m, 3 J=11.1Hz,1H,ArH),7.24–7.14(m,12H,ArH),7.10(m, 3 J=9.2Hz, 3H, ArH), 6.92(d, 4 J=1.6Hz,1H,ArH),6.83(s,1H,ArH),3.91(s,2H,ArCH 2 ),3.84(s,2H,NCH 2 C=N)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com