Synthesis method of compound with biphenyl dimer structure
A biphenyl dimer and synthesis method technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of low yield, difficult purification, high cost of raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
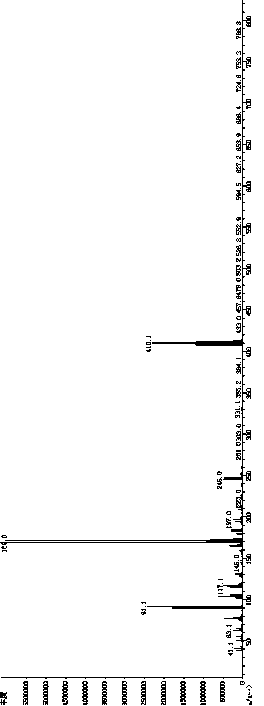
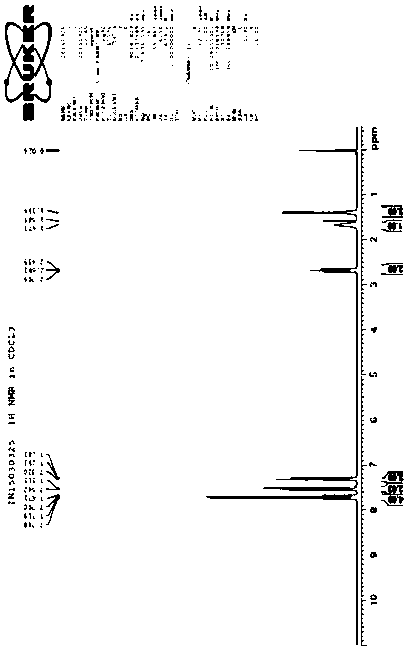
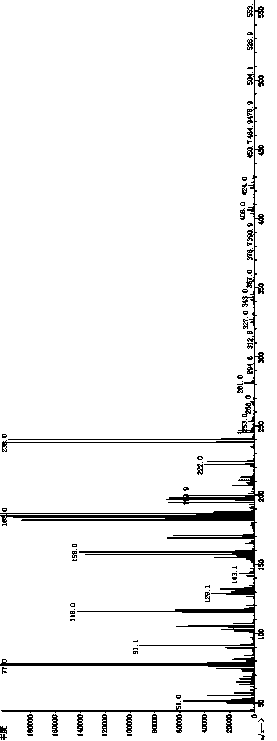
Image
Examples
Embodiment 1
[0035] 1) Add 192.5g Mg and 2g iodine into a 5L reaction flask, fully dissolve them in 2.5L tetrahydrofuran, and add dropwise 1 / 3 of 50mL 1,5-dibromo-pentane and 250mL tetrahydrofuran The mixed solution formed by mixing is heated to reflux after the reaction is initiated, and the remaining mixed solution is added dropwise, and the reaction is refluxed for 6 hours. The temperature is controlled at 35° C., and the mixed solution obtained by dissolving 250 g of p-bromo-benzaldehyde in 250 mL of tetrahydrofuran is added dropwise. Reaction for 3 hours;
[0036] After the reaction, add 700mL of dilute hydrochloric acid with a concentration of 10% dropwise to the reaction solution, adjust the pH of the reaction solution to below 2, and control the temperature of the reaction solution below 30°C, add 2L of water to dilute, and extract with ethyl acetate , combined the organic layers, and the organic layer was washed with saturated aqueous sodium bicarbonate until the pH was 6-7, then ...
Embodiment 2
[0040] 1) Add 192.5g Mg and 2g iodine into a 5L reaction flask, mix well with 2.5L tetrahydrofuran, and add dropwise 1 / 2 of 50mL 1,5-dibromo-pentane and 250mL tetrahydrofuran under the condition of nitrogen protection Mix the mixed solution formed, after initiating the reaction, heat up to reflux, add dropwise the remaining mixed solution, reflux reaction for 6 hours, control the temperature at 45°C, add dropwise the compound (4-bromobenzaldehyde ) The mixed solution obtained was reacted for 4.5 hours;
[0041] After the reaction, add 700mL of dilute hydrochloric acid with a concentration of 10% dropwise to the reaction solution, adjust the pH of the reaction solution to below 2, and control the temperature of the reaction solution below 30°C, add 2L of water to dilute, and extract with ethyl acetate , combined the organic layers, and the organic layer was washed with saturated aqueous sodium bicarbonate until the pH was 6-7, then washed with water until the pH was neutral, dr...
Embodiment 3
[0045]1) Add 144g Mg and 2g iodine into a 2.5L reaction flask, mix well with 1L ether, and add 1 / 3 of 12.3mL 1,5-dibromo-pentane and 65mL tetrahydrofuran dropwise under the condition of nitrogen protection The mixed solution formed by mixing is heated to reflux after the reaction is initiated, and the remaining mixed solution is added dropwise, and the reflux reaction is carried out for 6 hours. The temperature is controlled at 25°C, and the mixed solution obtained by dissolving 185g (4-bromobenzaldehyde) in 185mL tetrahydrofuran is added dropwise. Solution, reacted for 3 hours;
[0046] After the reaction was finished, 700 mL of dilute hydrochloric acid with a concentration of 10% was added dropwise to the reaction solution, the pH of the reaction solution was adjusted to below 2, and the temperature of the reaction solution was controlled to be 30° C., diluted with 1 L of water, extracted with ethyl acetate, The organic layers were combined, and the organic layer was washed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com