Method for treating organic waste acids in p-acetamidobenzene sulfonyl chloride synthesis
A technique for treating acetaminobenzenesulfonyl chloride and its treatment method, which is applied in the field of synthesis of acetaminobenzenesulfonyl chloride, and can solve the problems of difficult utilization of organic waste acids, high production and environmental protection treatment costs, and low yield of acetaminobenzenesulfonyl chloride.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
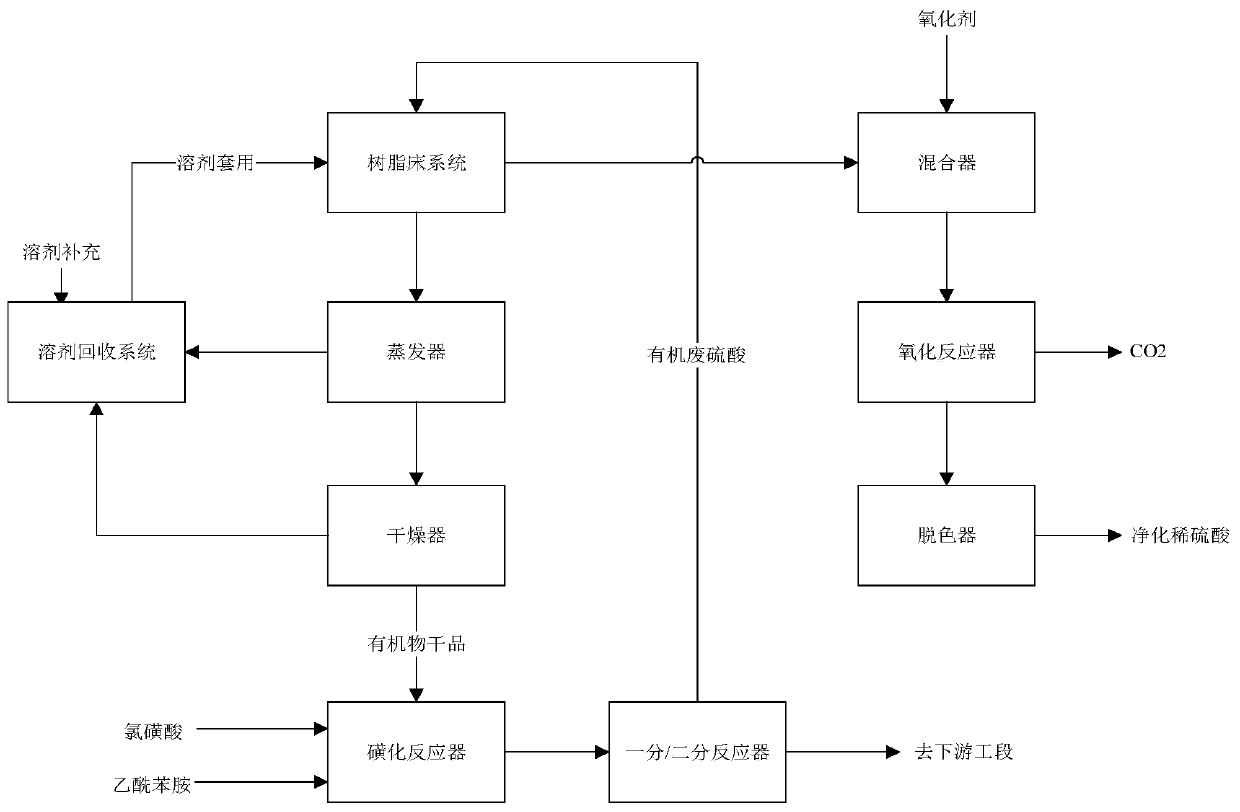
[0019] The present embodiment provides a kind of processing method of organic waste acid in the synthesis of acetaminobenzenesulfonyl chloride, and it comprises the following steps:
[0020] (a) Pump organic waste sulfuric acid (the organic waste sulfuric acid is tested, and the percentage of organic matter is 4.3%, the same below) is pumped into the macroporous resin bed, and organic matter is adsorbed at 20°C and a pressure of 1barg Retention, the hydraulic retention time is 15min; after testing, the organic waste sulfuric acid (defined as sulfuric acid solution) after being absorbed by the macroporous resin bed, the concentration of organic matter in the organic waste sulfuric acid is 0.12% (the organic matter adsorption removal rate is 97.2%);
[0021] (b) After the aforementioned sulfuric acid solution is pumped into the mixer and fully mixed with the oxidant (the amount of hydrogen peroxide added is 5% of the mass of the sulfuric acid solution, calculated as 27.5%wt), it ...
Embodiment 2
[0027] This example provides a treatment method for organic waste acid in the synthesis of acetaminobenzenesulfonyl chloride, which is basically the same as that in Example 1, except that in step (a), the parameters in the macroporous resin bed are controlled as The temperature is 0°C, 5 barg and the hydraulic retention time is 20 min; the yield of p-acetamidobenzenesulfonyl chloride is finally synthesized up to 96.5%.
Embodiment 3
[0029] This example provides a treatment method for organic waste acid in the synthesis of acetaminobenzenesulfonyl chloride, which is basically the same as that in Example 1, except that in step (a), the parameters in the macroporous resin bed are controlled as The temperature is 60°C, the normal pressure and the hydraulic retention time are 5 minutes; the yield of the final synthesis of acetaminobenzenesulfonyl chloride reaches 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com