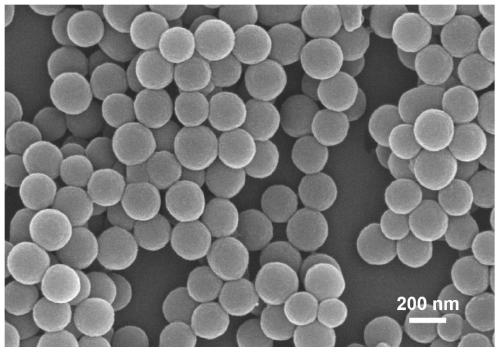
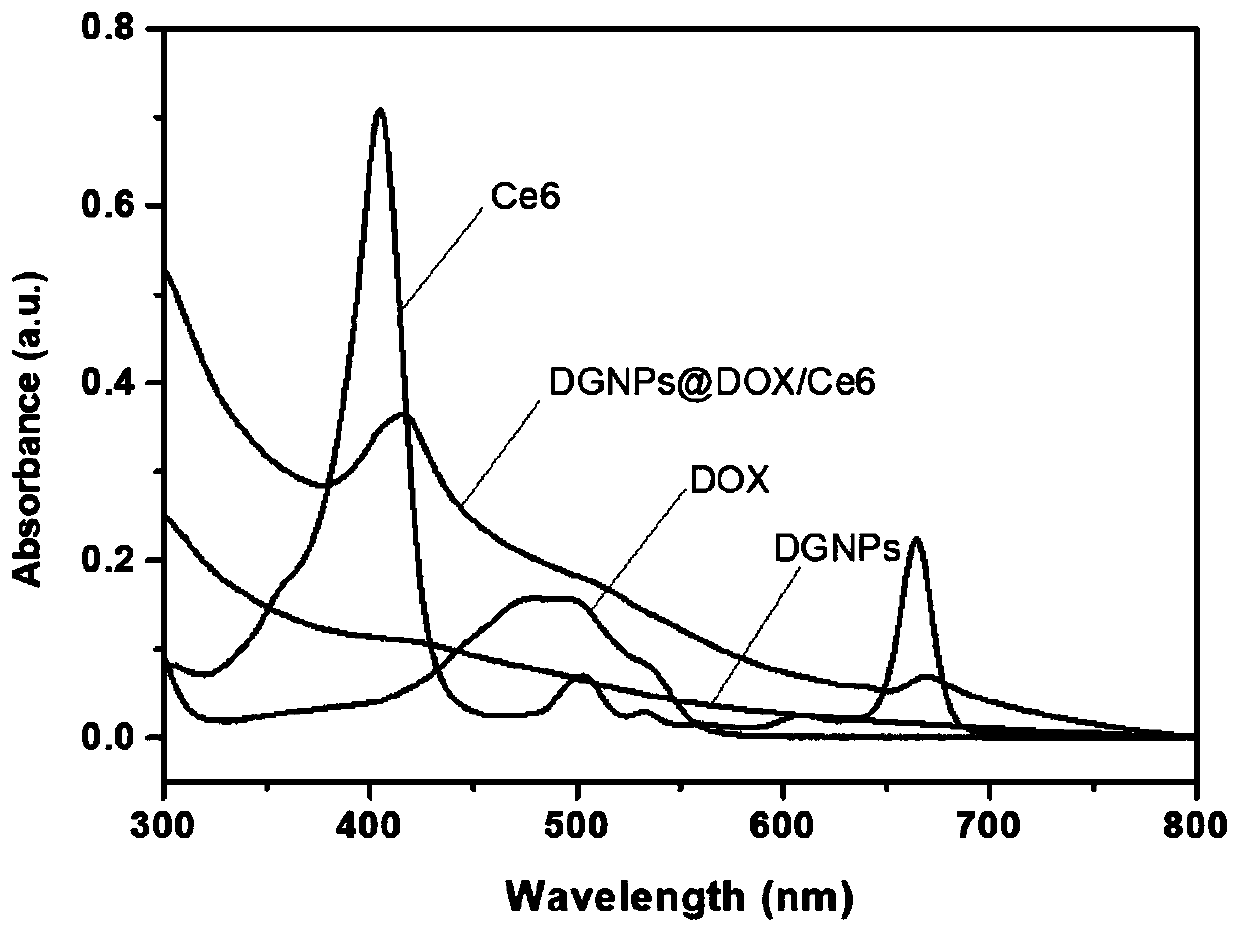
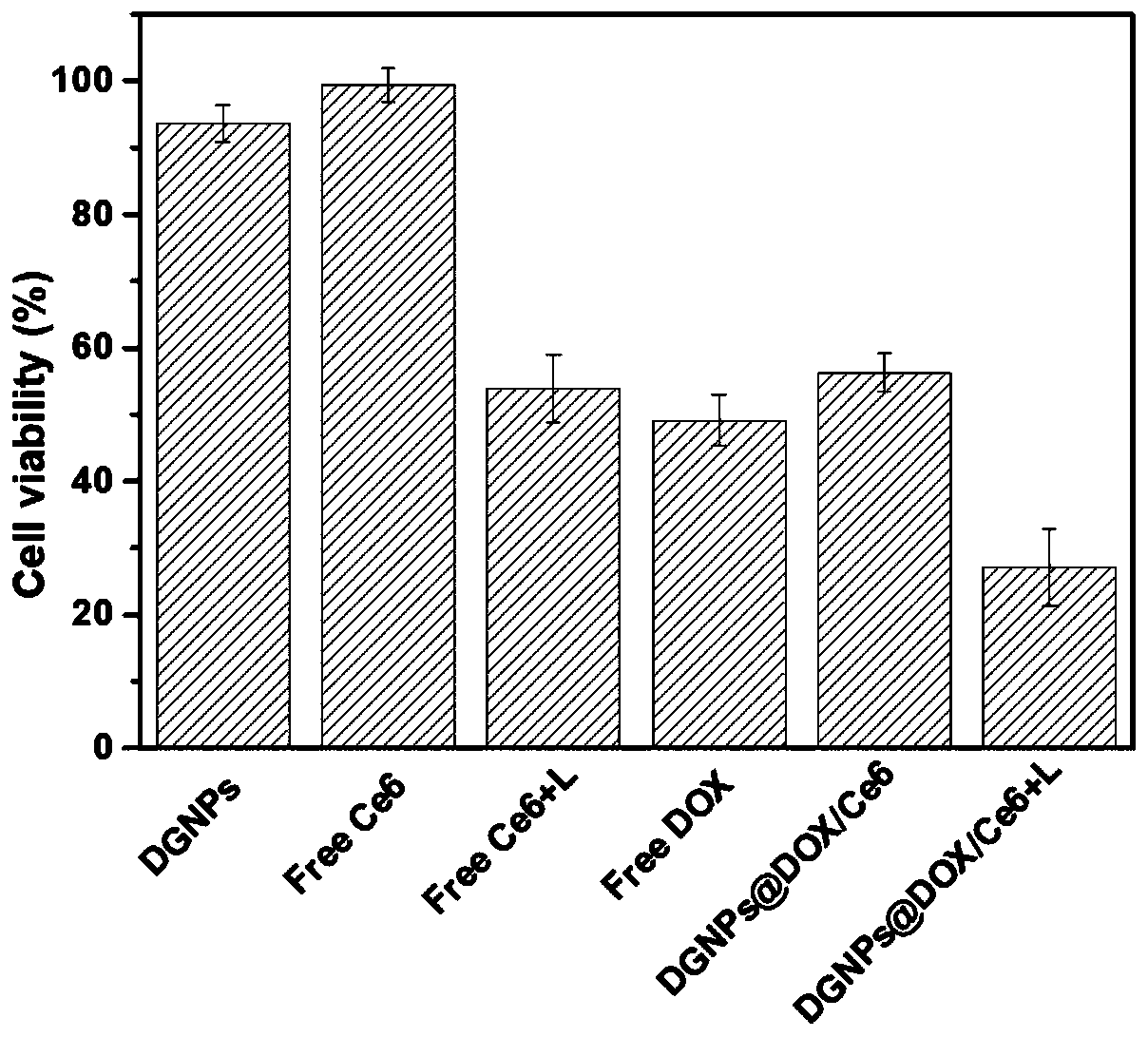
Dopamine assembly medicine delivery system and preparation method thereof
A delivery system and assembly technology, which is applied in the field of new dopamine assembly drug delivery system and its preparation, to achieve the effect of optimizing the therapeutic effect and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Dissolving dopamine hydrochloride in a PBS buffer solution with a pH of 7.4 to prepare a dopamine hydrochloride solution with a concentration of 3 mM; dissolving glutaraldehyde in a PBS buffer solution with a pH of 7.4 to prepare a mass concentration of 0.06% glutaraldehyde solution; then according to the mass ratio of dopamine hydrochloride and glutaraldehyde is 1:2, the dopamine hydrochloride solution of 0.5mL 3mM and 0.5mL mass concentration are 0.06% glutaraldehyde The solution was shaken and mixed evenly, and the pH of the mixed solution was adjusted to 6.7 with hydrochloric acid and sodium hydroxide aqueous solution, and aged at room temperature for 2 hours to obtain a dopamine-glutaraldehyde assembly solution.
[0026] 2. Add 50 μL of 1 mg / mL adriamycin aqueous solution and 50 μL of 1 mg / mL chlorin e6 solution to the obtained dopamine and glutaraldehyde assembly solution obtained in step 1 (by 10 mg / mL of chlorin e6DMSO The solution was diluted with deionized ...
Embodiment 2
[0028] 1. Dissolve dopamine hydrochloride in a PBS buffer solution with a pH of 7.4 to prepare a dopamine hydrochloride solution with a concentration of 6 mM; dissolve oxidized sodium alginate in a PBS buffer solution with a pH of 7.4 to prepare a mass concentration 0.06% oxidized sodium alginate solution; then according to the molar ratio of dopamine hydrochloride and oxidized sodium alginate is 10:1, the dopamine hydrochloride solution of 6mM and the mass concentration are 0.06% oxidized sodium alginate The solution was oscillated and mixed evenly, and the total volume was 1 mL. The pH of the mixed solution was adjusted to 7.4 with hydrochloric acid and sodium hydroxide aqueous solution, and aged at room temperature for 4 hours to obtain a solution of dopamine and oxidized sodium alginate assembly.
[0029] 2. Add 50 μL of 1 mg / mL doxorubicin aqueous solution and 50 μL of 1 mg / mL chlorin e6 solution to the obtained dopamine and oxidized sodium alginate assembly solution obtai...
Embodiment 3
[0031] 1. Dissolving dopamine hydrochloride in a PBS buffer solution with a pH of 7.4 is prepared into a dopamine hydrochloride solution with a concentration of 8 mM; dissolving glutaraldehyde in a PBS buffer solution with a pH of 7.4 is prepared with a mass concentration of 0.2% glutaraldehyde solution; then according to the molar ratio of dopamine hydrochloride and glutaraldehyde is 2:5, the dopamine hydrochloride solution of 0.5mL 8mM and 0.5mL mass concentration are 0.2% glutaraldehyde The solution was shaken and mixed evenly, and the pH of the mixed solution was adjusted to 6.7 with hydrochloric acid and sodium hydroxide aqueous solution, and aged at room temperature for 4 hours to obtain a dopamine-glutaraldehyde assembly solution.
[0032] 2. Add 50 μL of 1 mg / mL bortezomib aqueous solution and 50 μL of 1 mg / mL phthalocyanine aqueous solution to the dopamine and glutaraldehyde assembly solution obtained in step 1, react under shaking at room temperature for 20 hours, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com