Method for recycling phosphorus pentafluoride in lithium hexafluorophosphate synthesis tail gas
A technology of lithium hexafluorophosphate and phosphorus pentafluoride, which is applied in lithium hexafluorophosphate, chemical instruments and methods, lithium compounds, etc., and can solve the problems of restricting industrial reuse and difficulty in separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
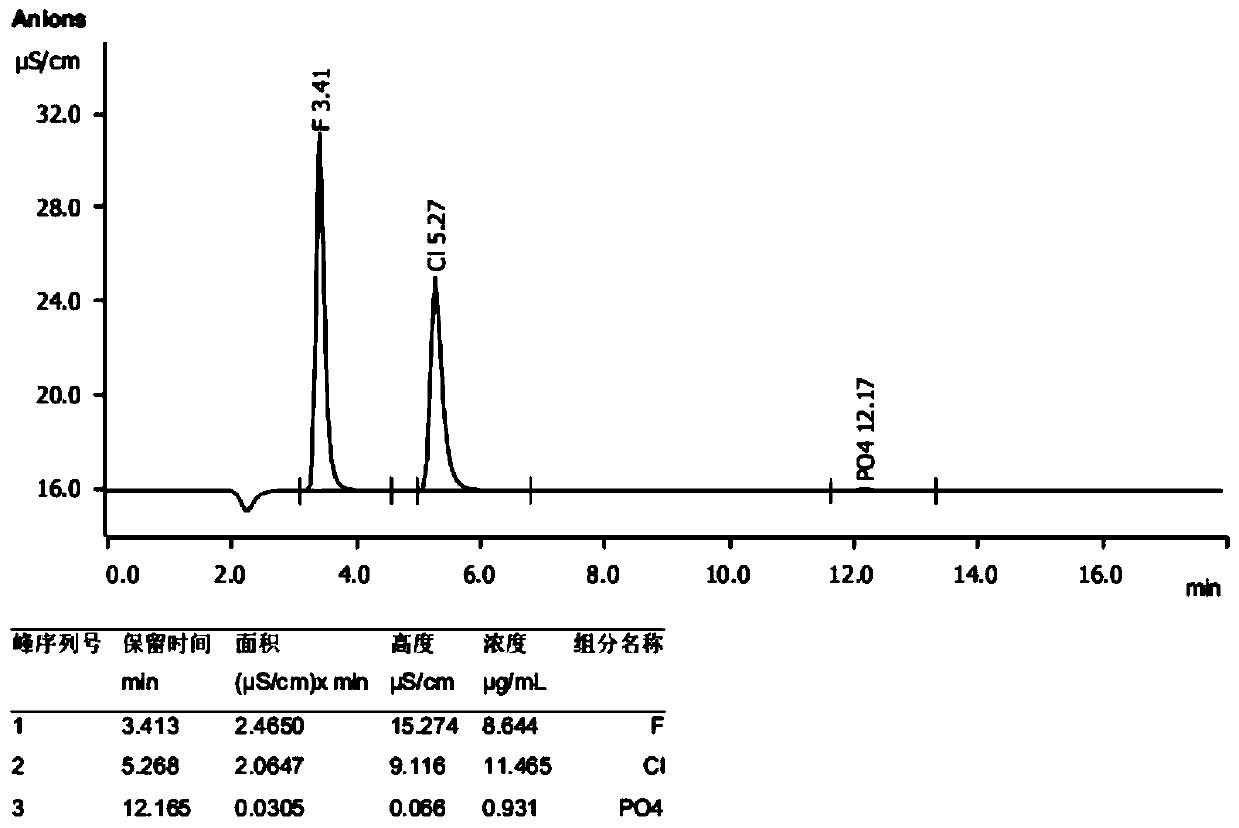
[0029] The tail gas of lithium hexafluorophosphate synthesis is a mixed gas of hydrogen fluoride, hydrogen chloride and phosphorus pentafluoride. Take a certain volume of mixed gas to measure the content of hydrogen fluoride, hydrogen chloride and phosphorus pentafluoride. The test method is as follows: absorb with three-stage sodium hydroxide lye, the size of the absorption tower is 40mm*300mm (inner diameter*height), and the packing of the absorption tower is uniform It is made of PTFE, Pall ring packing 5mm*φ5mm, the concentration of sodium hydroxide is 2% (w / w), and the alkali absorption solution is combined to determine F by ion chromatography - , Cl - and PO 4 3- The contents were 8.664μg / ml, 11.465μg / ml, 0.931μg / ml ( figure 1 ), according to a molecule of PF 5 After the lye is absorbed, a molecule of PO is produced 4 3- And the quantitative relationship of 5 molecules HF is converted into mass ratio, HF, HCl and PF 5 The mass ratio is 0.552 / 0.384 / 0.064 (w / w).
...
Embodiment 2
[0032]The ratio of hydrogen chloride, hydrogen fluoride and phosphorus pentafluoride in the tail gas of lithium hexafluorophosphate synthesis HCl: HF: PF 5 =0.552:0.384:0.064 (w / w) (the measurement method is the same as that in Example 1). The mixed gas passes through the scrubber made of PTFE at a flow rate of 20mL / min. The internal size of the scrubber is 40mm*300mm (inner diameter*height), filled with 10*10 Pall ring fillers, and the absorbent is n-heptene (excess ), the operating temperature is 20°C. The gas that comes out from scrubber passes through three-stage sodium hydroxide alkali absorption tower, and the size of absorption tower is the same as aforementioned scrubber, and the concentration of sodium hydroxide is 2% (w / w), and ion chromatography is used after the alkali washing solution merges Determination of Cl - , F - and PO 4 3- The content is converted into HCl / HF / PF according to the mass ratio 5 = 0.571 / 0.406 / 0.023, PF 5 The absorption efficiency is 64....
Embodiment 3
[0034] The operating temperature of the scrubber is 10°C, and other operations are the same as in Example 1. After testing, PF 5 The absorption efficiency is 32.4%. The absorption rate of this embodiment is obviously lower than that of Example 1, indicating that the operating temperature of the scrubber is very critical, and too low temperature will reduce the absorption rate of phosphorus pentafluoride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com