Strontium-promoted cobalt-based composite oxide catalysts for hydrogen production by autothermal reforming of acetic acid
A cobalt-based catalyst and autothermal reforming technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical elements of heterogeneous catalysts, etc., can solve the problem of carbon deposition and catalyst deactivation And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] Weigh 3.574g of Sr(NO 3 ) 2 , 2.949g of Co(NO 3 ) 2 ·6H 2 O and 6.283g of Ce(NO 3 ) 3 ·6H 2 O, add 20.0mL of deionized water to prepare a mixed solution; weigh 1.802g of anhydrous glucose, dissolve it in 100mL of deionized water, and prepare a 0.1mol / L glucose solution, and weigh 8.719g of citric acid to prepare a solution ; Add citric acid and 10mL glucose solution to the nitrate mixed solution in turn, stir well; add ammonia water (28wt.%) solution dropwise, adjust the pH value of the mixed solution to about 4.2, and keep stirring for 1-2h, The solution was diluted to a total volume of 100.0mL, transferred to a 100mL autoclave, and placed in an oven at 170°C for hydrothermal treatment for 20h; after the reaction, when the autoclave was naturally cooled to room temperature, the resulting mixture was suction filtered and the precipitate was washed to neutral, and then dried in an oven at 105°C for 12 hours to obtain a catalyst precursor; the dried sample was calc...
Embodiment 1
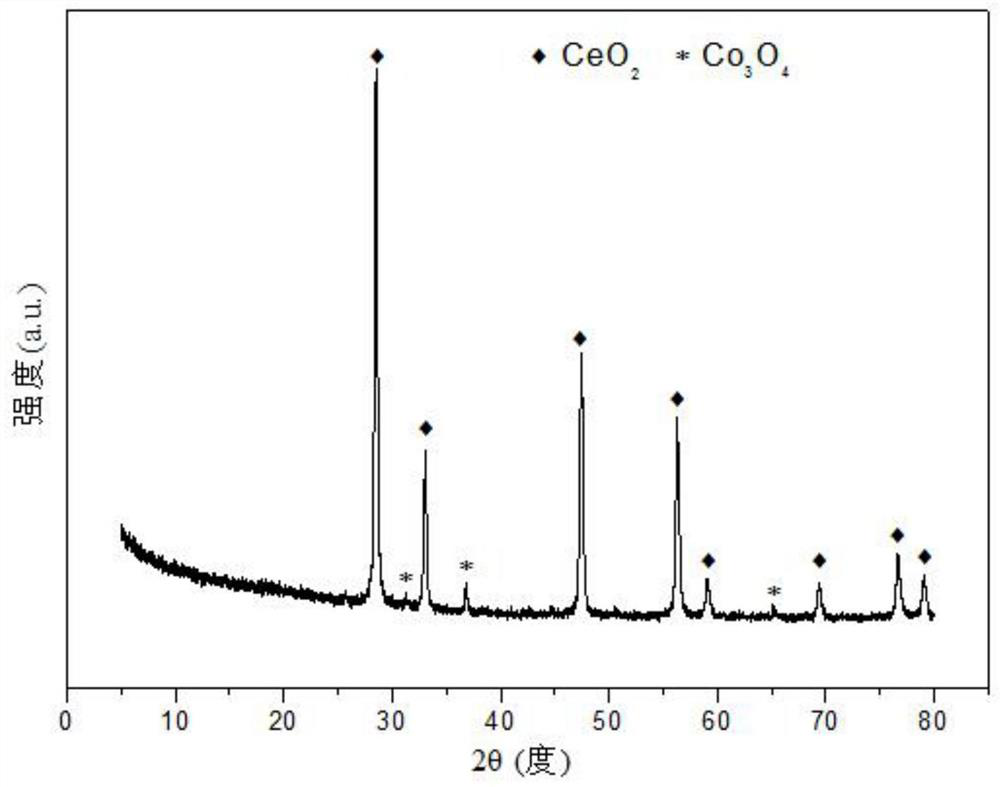
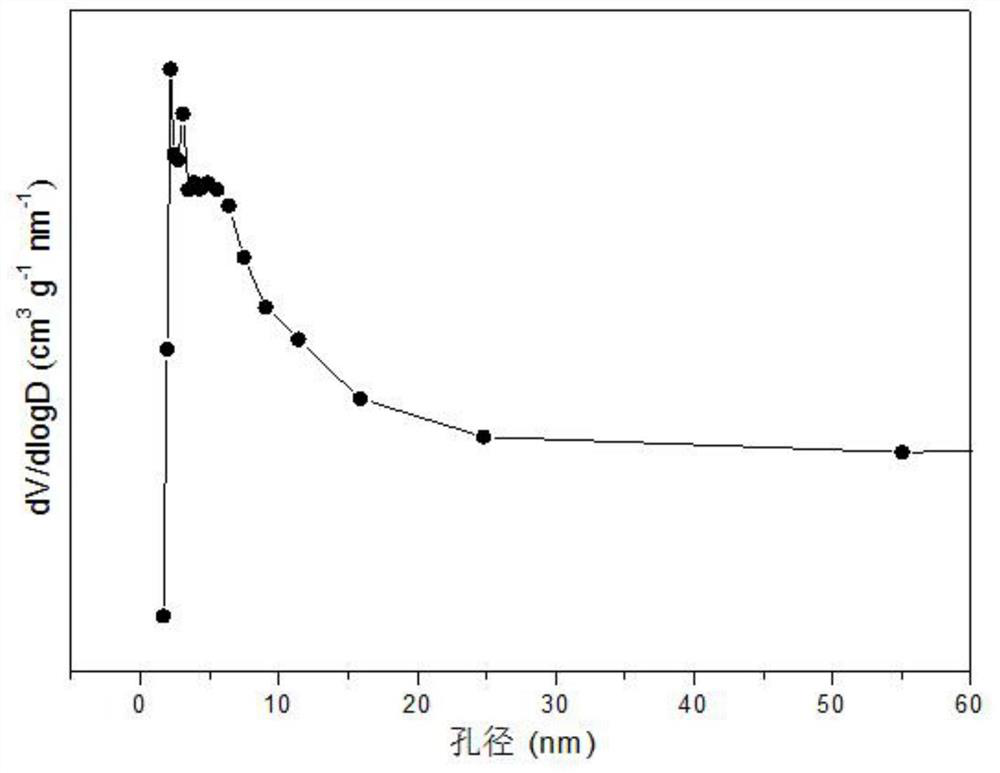
[0030] Weigh 1.336g of Sr(NO 3 ) 2 , 2.917g of Co(NO 3 ) 2 ·6H 2 O and 9.133g of Ce(NO 3 ) 3 ·6H 2 O, add 20.0mL of deionized water to prepare a mixed solution; weigh 1.802g of anhydrous glucose, dissolve it in 100mL of deionized water to prepare a 0.1mol / L glucose solution, and weigh 7.781g of citric acid to prepare a solution ; Add citric acid and 10mL glucose solution to the nitrate mixed solution successively, and stir evenly; Subsequent steps are the same as in Reference Example 1 to obtain the catalyst precursor, and after roasting, a strontium-promoted cobalt-based catalyst supported by cerium oxide is obtained. structure as attached figure 1 As shown, the catalyst presents ceria crystals as the skeleton, strontium oxide enters ceria in an amorphous form to form a Sr-Co-O solid solution, and contains a small amount of spinel phase of tricobalt tetroxide, which is highly dispersed on the solid solution. The pore size distribution of its typical mesoporous structu...
Embodiment 2
[0033] Weigh 2.378g of Sr(NO 3 ) 2 , 2.878g of Co(NO 3 ) 2 ·6H 2 O and 7.805g of Ce(NO 3 ) 3 ·6H 2 O, add 20.0mL of deionized water to prepare a mixed solution; weigh 1.802g of anhydrous glucose, dissolve it in 100mL of deionized water to prepare a 0.1mol / L glucose solution, and weigh 8.218g of citric acid to prepare solution; citric acid and 10mL glucose solution were added successively to the nitrate mixed solution, and stirred evenly; the subsequent steps were the same as in Reference Example 1 to obtain the catalyst precursor, and after roasting, the strontium-promoted cobalt-based catalyst supported by ceria was obtained, namely Obtain CDUT-SCC-103 catalyst, the chemical composition of this catalyst is (SrO) 1.25 (CoO 1.5 ) 1.10 (CeO 2 ) 2.00 , The weight percentage is the composition: strontium oxide is 23.3%, cobalt oxide is 14.8%, and cerium oxide is 61.9%.
[0034] The catalyst CDUT-SCC-103 was investigated for its activity in the autothermal reforming of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com