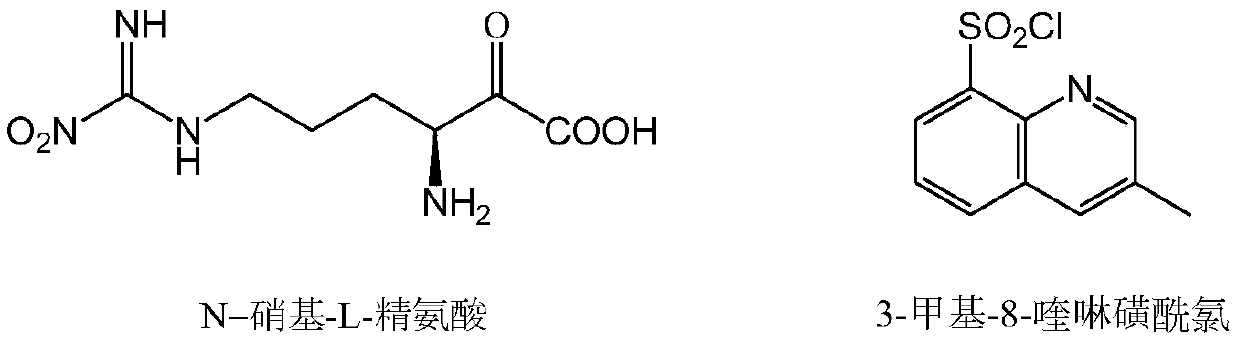
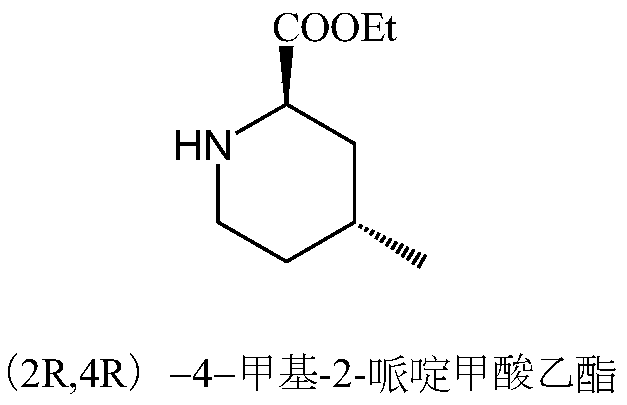
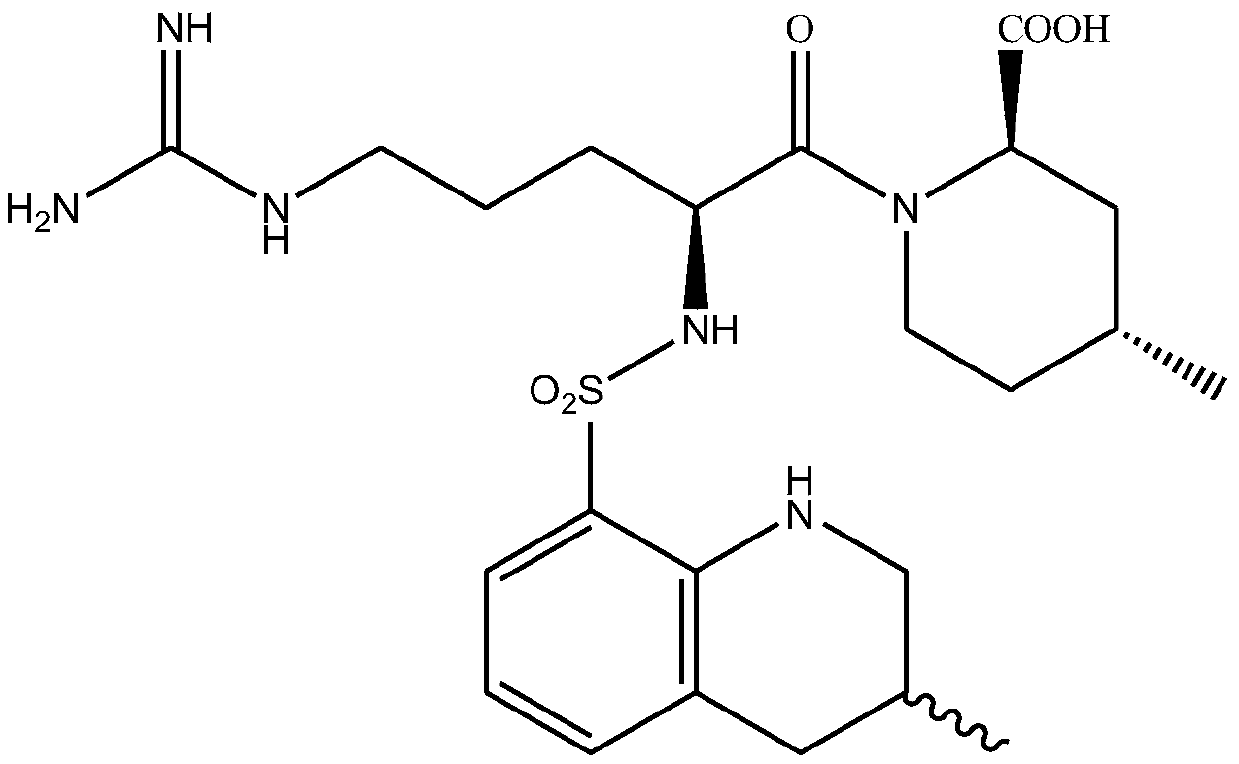
Method for continuously preparing argatroban
A technology of argatroban and dripping, which is applied in the field of medicine, can solve problems such as high temperature and high pressure reaction time, and achieve the effects of reducing the amount of condensing agent, reducing production costs, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The method for the continuous preparation of argatroban of embodiment 1
[0053] (1) Sulfonylation reaction
[0054] Add 25.8g (0.645mol) of sodium hydroxide and 400ml of water to a 1000mL three-necked flask, stir to dissolve, then add 50g (0.43mol) N-nitro-L-arginine and stir to dissolve at room temperature; in another 500ml three-necked flask Add 135.1g (0.559mol) of 3-methyl-8-quinolinesulfonyl chloride and 250ml of chloroform, stir to dissolve, and transfer to the above-mentioned 1000ml three-neck flask. After the transfer is complete, add 0.5g TEBA, raise the temperature to 40°C for 2 hours, let stand to separate the layers, and discard the organic phase. Add 400ml of chloroform to the water phase, adjust the pH to 6.0 with concentrated hydrochloric acid, stir, separate layers, and discard the water phase. Add 40 g of anhydrous sodium sulfate to the organic phase, dry for 30 min, filter, and transfer the filtrate to a 1000 ml three-necked flask, and transfer to t...
Embodiment 2
[0063] The method for the continuous preparation of argatroban of embodiment 2
[0064] (1) Sulfonylation reaction
[0065] Add 24.2g (0.43mol) of potassium hydroxide and 250ml of water to a 1000mL three-necked flask, stir to dissolve, then add 50g (0.43mol) N-nitro-L-arginine and stir to dissolve at room temperature; in another 500ml three-necked flask Add 103.9g (0.43mol) of 3-methyl-8-quinolinesulfonyl chloride and 150ml of dichloromethane, stir to dissolve, and transfer to the above-mentioned 1000ml three-necked flask. After the transfer was completed, 0.25 g of tetrabutylammonium bromide was added, the temperature was raised to 30° C. for 3 h, the mixture was allowed to stand and the layers were separated, and the organic phase was discarded. Add 250ml of dichloromethane to the water phase, adjust the pH to 5.0 with concentrated hydrochloric acid, stir, separate layers, and discard the water phase. Add 30 g of anhydrous magnesium sulfate to the organic phase, dry it for...
Embodiment 3
[0074] The method for the continuous preparation of argatroban of embodiment 3
[0075] (1) Sulfonylation reaction
[0076]Add 31.6g (0.559mol) of potassium hydroxide and 300ml of water to a 1000mL three-necked flask, stir to dissolve, then add 50g (0.43mol) N-nitro-L-arginine and stir to dissolve at room temperature; in another 500ml three-necked flask Add 124.7g (0.516mol) of 3-methyl-8-quinolinesulfonyl chloride and 200ml of dichloromethane, stir to dissolve, and transfer to the above-mentioned 1000ml three-necked flask. After the transfer is complete, add 0.4g TEBA, raise the temperature to 40°C for 2 hours, let stand to separate the layers, and discard the organic phase. Add 300ml of dichloromethane to the water phase, adjust the pH to 5.4 with concentrated hydrochloric acid, stir, separate layers, and discard the water phase. Add 35g of anhydrous magnesium sulfate to the organic phase, dry for 30min, filter, transfer the filtrate into a 1000ml three-neck flask, and tra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com