Stable isotope labeled norfloxacin and synthesis method thereof
A technology of stable isotope and synthesis method, which is applied in the field of stable isotope-labeled norfloxacin and its synthesis, can solve the problems of low overall yield, high reaction temperature, low yield and the like, and achieves simple synthesis process, easy separation and purification , the effect of high atomic utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
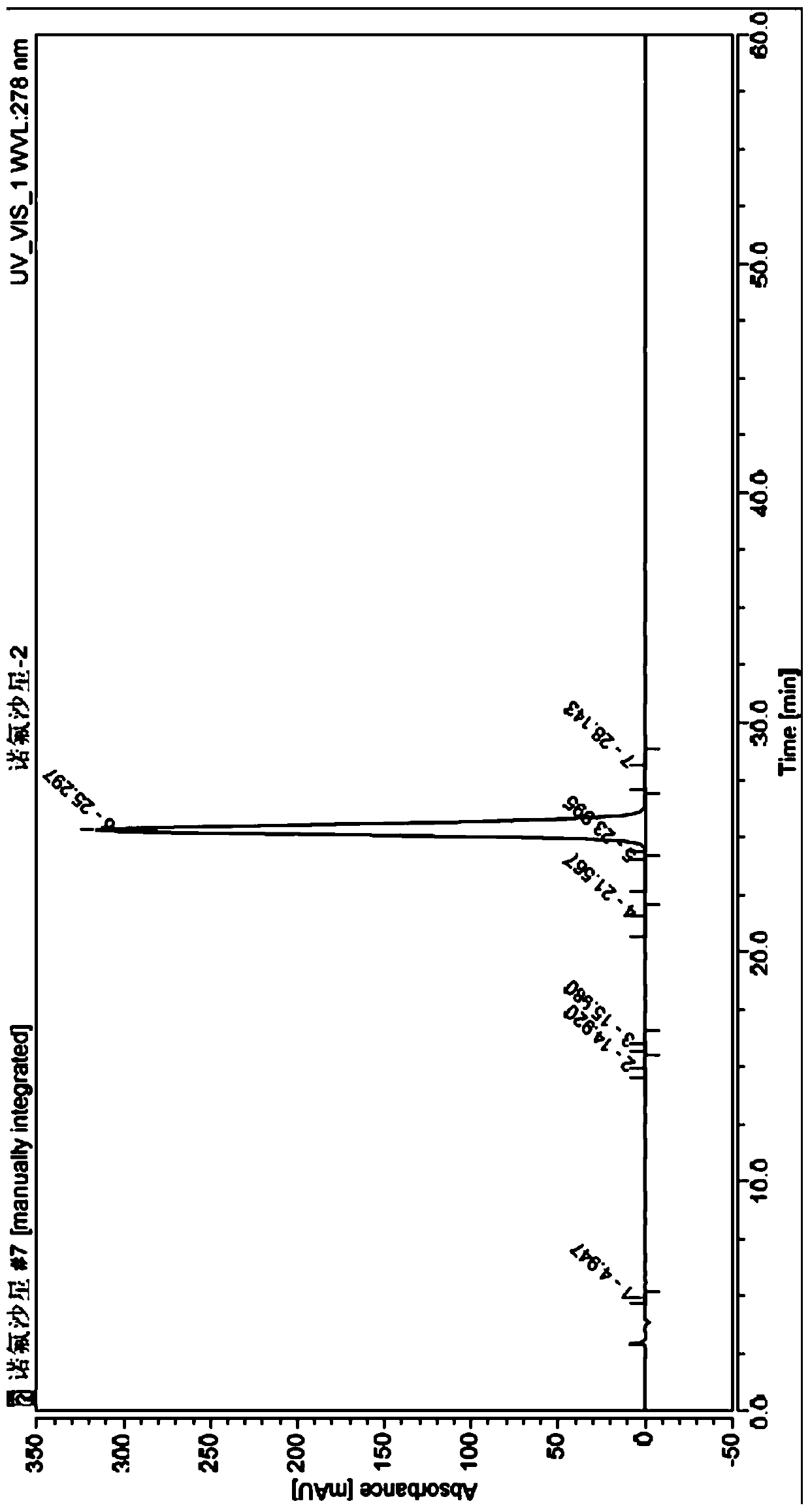
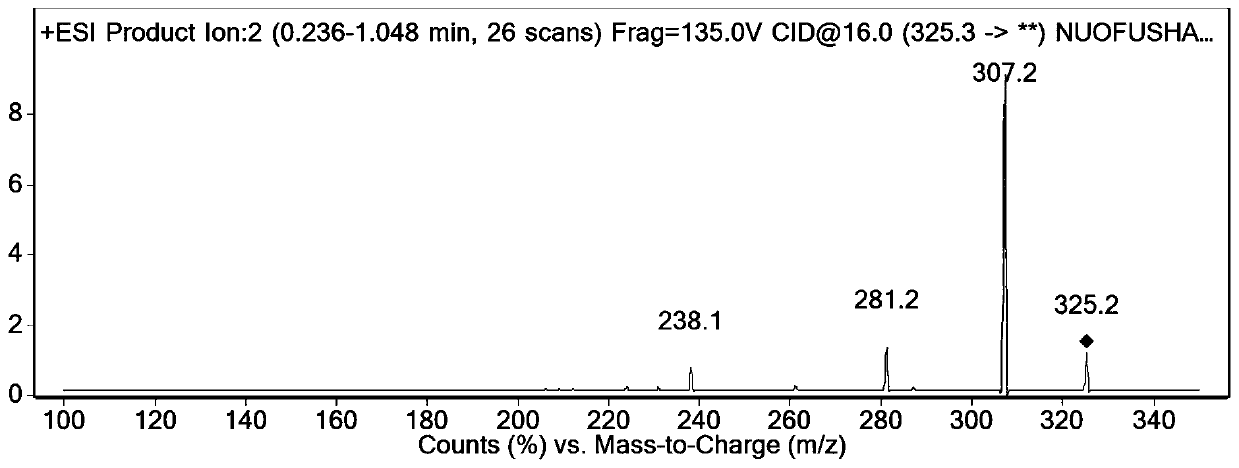
Image
Examples
preparation example Construction
[0021] The synthetic method of the norfloxacin of stable isotope label provided by the invention comprises the following steps:
[0022] S1: reacting 3,4-difluoroaniline with diethyl ethoxymethylene malonate to obtain diethyl 3,4-difluoroaniline methylene malonate;
[0023] Described molecular structure is:
[0024]
[0025] S2: The 3,4-difluoroaniline methylene malonate diethyl ester is subjected to a ring closure reaction in a diphenyl ether solution to obtain 6,7-difluoro-4-hydroxyquinoline-3-carboxylic acid ethyl ester;
[0026] Described molecular structure is:
[0027]
[0028] S3: Using ethyl 6,7-difluoro-4-hydroxyquinoline-3-carboxylate to react with stable isotope-labeled bromoethane to obtain stable isotope-labeled 6,7-difluoro-1-ethyl-1 , ethyl 4-dihydro-4-oxoquinoline-3-carboxylate;
[0029] The molecular structure of described stable isotope-labeled ethyl bromide is:
[0030]
[0031] The molecular structure of the stable isotope label is:
[0032] ...
Embodiment 1
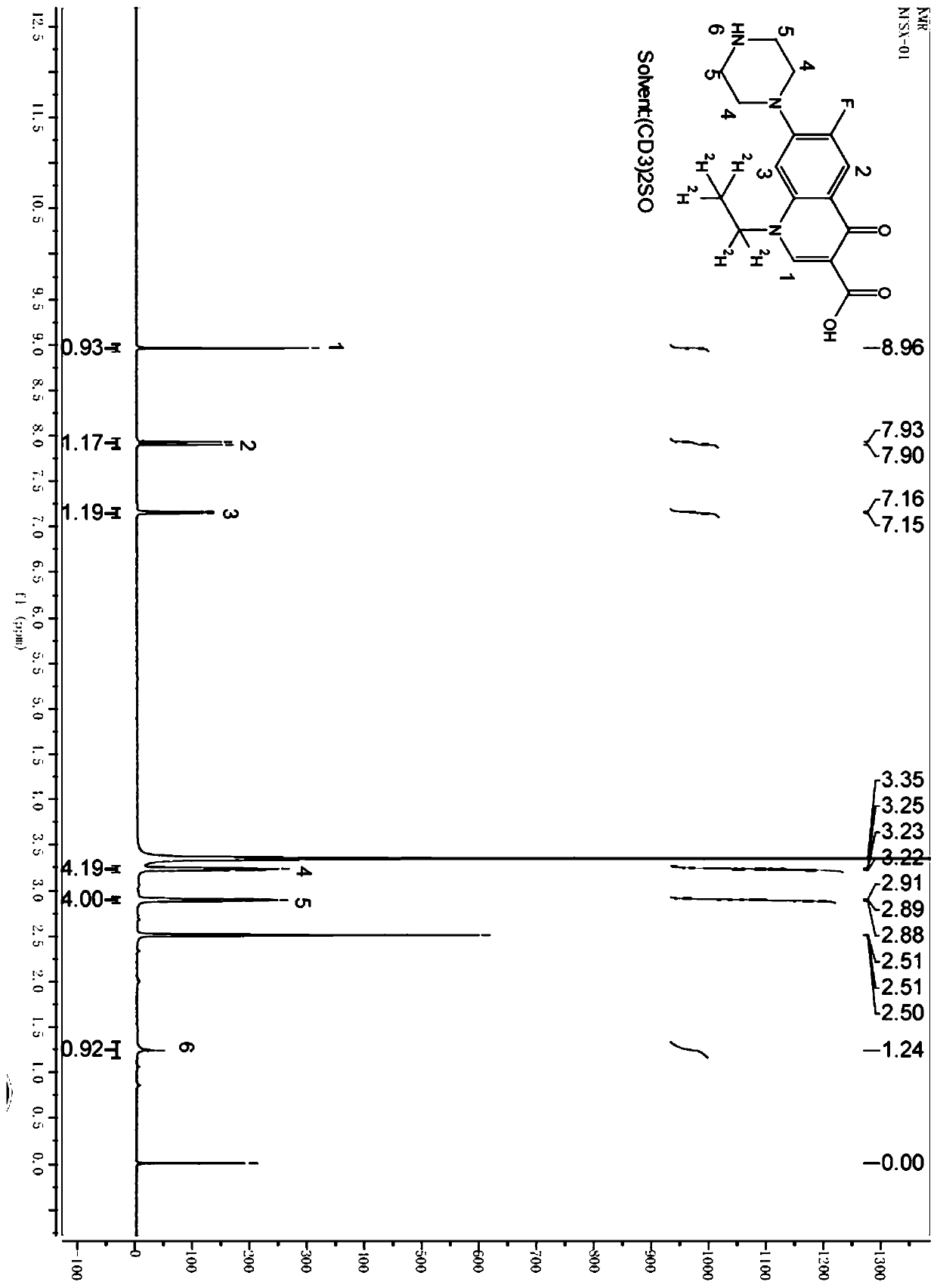
[0040] The molecular structure of stable isotope-labeled norfloxacin is as follows:
[0041]
[0042] Prepared by the following synthetic steps:
[0043] S1. Add 20g of 3,4-difluoroanilinomorpholine and 33.5g of diethyl ethoxymethylene malonate into the reaction vessel, and keep it warm at 120-130°C for 3 hours, then stop the heating of the reaction vessel, and naturally Cool, filter, and the filtered solid can be directly used in the next step of synthesis without further purification.
[0044] S2. Add 45g of 3,4-difluoroanilinomethylene diethyl malonate to the reaction vessel, add 350ml of diphenyl ether, and react at a temperature of 240-280°C for 2 hours. After the reaction is completed, cool to room temperature, filter out solids.
[0045] S3. Add 30g of 6,7-difluoro-4-hydroxyquinoline-3-carboxylic acid ethyl ester to the reaction vessel, add 150ml of N,N-dimethylformamide, add 32.8g of anhydrous potassium carbonate, add dropwise 15g Stable isotope-labeled ethyl bro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com