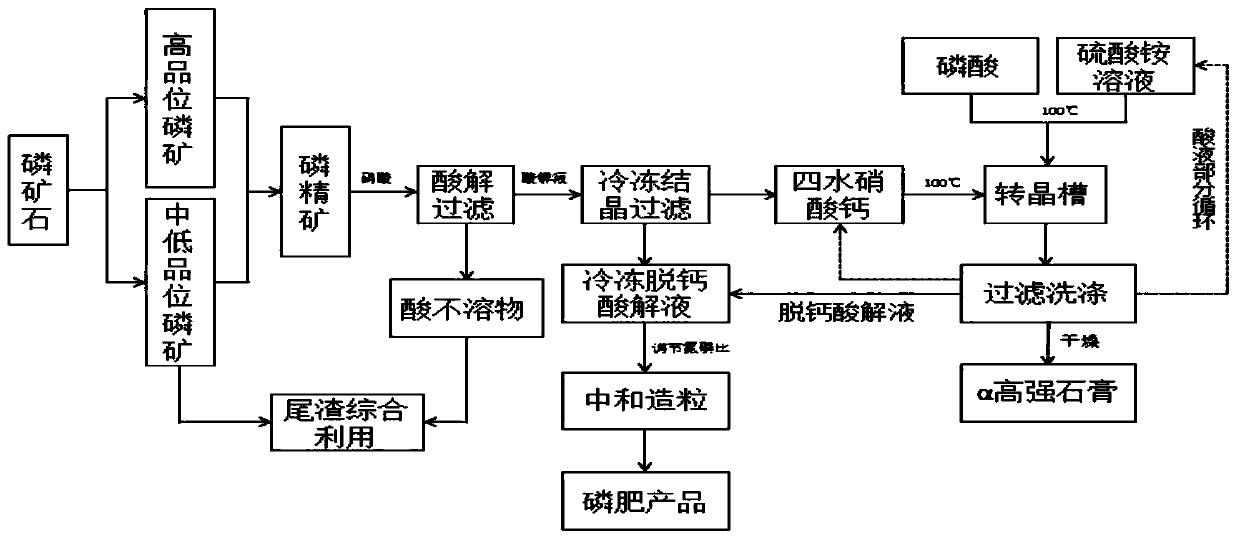
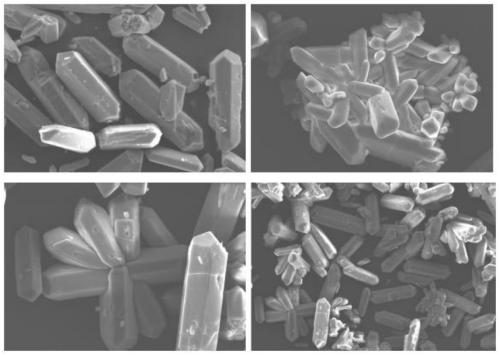
Method for preparing alpha high-strength gypsum from freezing method nitrophosphate byproduct calcium nitrate tetrahydrate
A technology of calcium nitrate tetrahydrate and nitrophosphate fertilizer, applied in chemical instruments and methods, calcium/strontium/barium nitrate, calcium/strontium/barium compounds, etc., can solve long process, difficulty in freezing and separating calcium nitrate, complicated operation, etc. problems, to achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, implementation raw materials: 1, phosphate concentrate powder: P205 content is 31.12%, CaO is 46.23%; 2, nitric acid; 3, ammonium sulfate solution; 4, phosphoric acid.
[0042] A method for making α high-strength gypsum by-product calcium nitrate tetrahydrate by freezing nitrophosphate fertilizer, comprising the following steps:
[0043] (1) Acid hydrolysis: acidolysis reaction is carried out with nitric acid after the phosphate concentrate powder is added water, and the mass concentration of nitric acid is 60%; The acid-mineral ratio of nitric acid and phosphate concentrate is 1.25:1, and the acid excess coefficient of nitric acid is 120%; The temperature of the acidolysis reaction is 60°C, and the time of the acidolysis reaction is 3 hours to obtain a crude acidolysis solution;
[0044] (2) Filtration: the crude acid solution of step (1) is filtered to remove acid insolubles and organic matter, to obtain the acid solution;
[0045] (3) freezing and crys...
Embodiment 2
[0050] Embodiment 2, implementation raw materials: 1, phosphate concentrate powder: P205 content is 31.12%, CaO is 46.23%; 2, nitric acid; 3, ammonium sulfate; 4, phosphoric acid.
[0051] A method for making α high-strength gypsum by-product calcium nitrate tetrahydrate by freezing nitrophosphate fertilizer, comprising the following steps:
[0052] (1) Acid hydrolysis: acid hydrolysis reaction is carried out with nitric acid after the phosphate concentrate powder is added water, and the mass concentration of nitric acid is 50%; The acid-mineral ratio of nitric acid and phosphate concentrate is 1.3:1, and the acid excess coefficient of nitric acid is 115%; The temperature of the acidolysis reaction is 50°C, and the time of the acidolysis reaction is 2 hours to obtain a crude acidolysis solution;
[0053] (2) Filtration: the crude acid solution of step (1) is filtered to remove acid insolubles and organic matter, to obtain the acid solution;
[0054] (3) freeze crystallization...
Embodiment 3
[0059] Embodiment 3, implementation raw materials: 1, phosphate concentrate powder: P205 content is 31.12%, CaO is 46.23%; 2, nitric acid; 3, ammonium sulfate; 4, phosphoric acid.
[0060] A method for making α high-strength gypsum by-product calcium nitrate tetrahydrate by freezing nitrophosphate fertilizer, comprising the following steps:
[0061] (1) Acid hydrolysis: acid hydrolysis reaction is carried out with nitric acid after the phosphate concentrate powder is added water, and the mass concentration of nitric acid is 50%; The acid-mineral ratio of nitric acid and phosphate concentrate is 1.2:1, and the acid excess coefficient of nitric acid is 105%; The temperature of the acidolysis reaction is 40°C, and the time of the acidolysis reaction is 1 hour to obtain a crude acidolysis solution;
[0062] (2) Filtration: the crude acid solution of step (1) is filtered to remove acid insolubles and organic matter, to obtain the acid solution;
[0063] (3) freezing and crystalliz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
| flexural strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com