Cefoperazone compound pharmaceutical preparations and new indications for the treatment of endometritis and other gynecological reproductive tract infections
A cefoperazone sodium, reaction technology, applied in the field of drug preparation, can solve problems such as allergic reaction, polymer increase, pharmacological hazards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
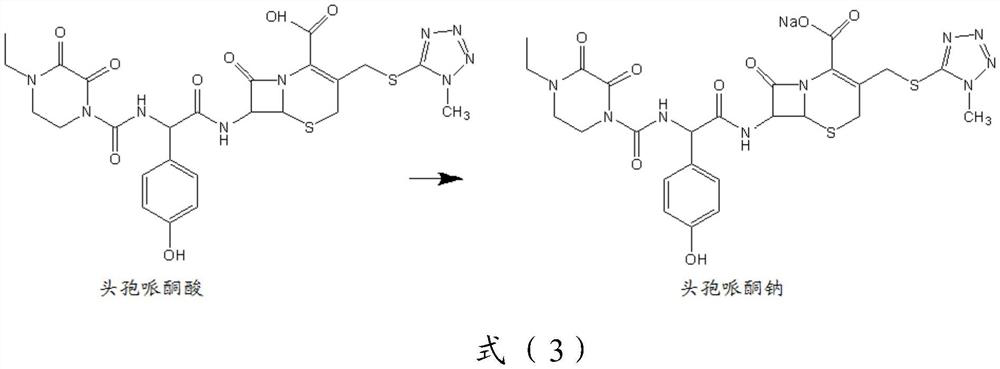
[0108] The synthesis of embodiment 1 cefoperazone sodium
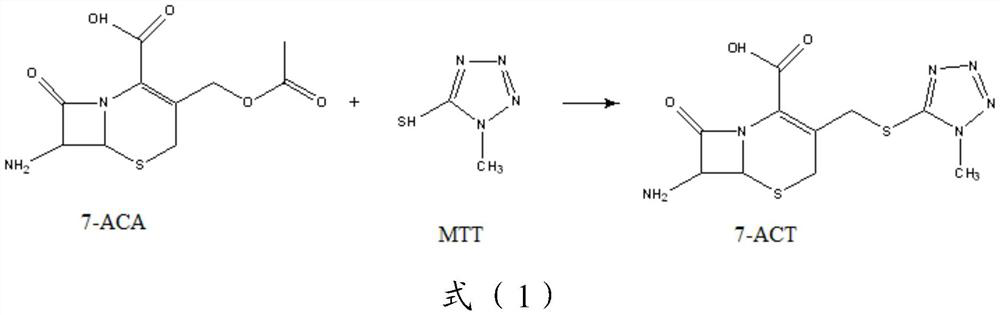
[0109] Step 1), 272g (1.0mol, M=272.28) 7-ACA, 122g (1.05mol, M=116.14) MTT were dissolved in acetonitrile (820mL) containing 83.1g pyridine (1.05mol, M=79.10), cooling to 5°C, add 208.6g TiCl 4 (1.1 mol, M=189.68)—37.4 g tetrabutyl titanate (0.11 mol, M=340.3) catalyst, heat up to 30° C., and react for 3 h under stirring. After the reaction, filter, cool the filtrate to 0-5°C, add 10% ammonia water dropwise to the isoelectric point under stirring, crystallization occurs, continue to stir for 1h, filter with suction, wash with acetone, and dry to obtain a white solid powder 7-ACT ( M=328.37) 327.4g, yield 98.0%, HPLC content greater than 98.3%.
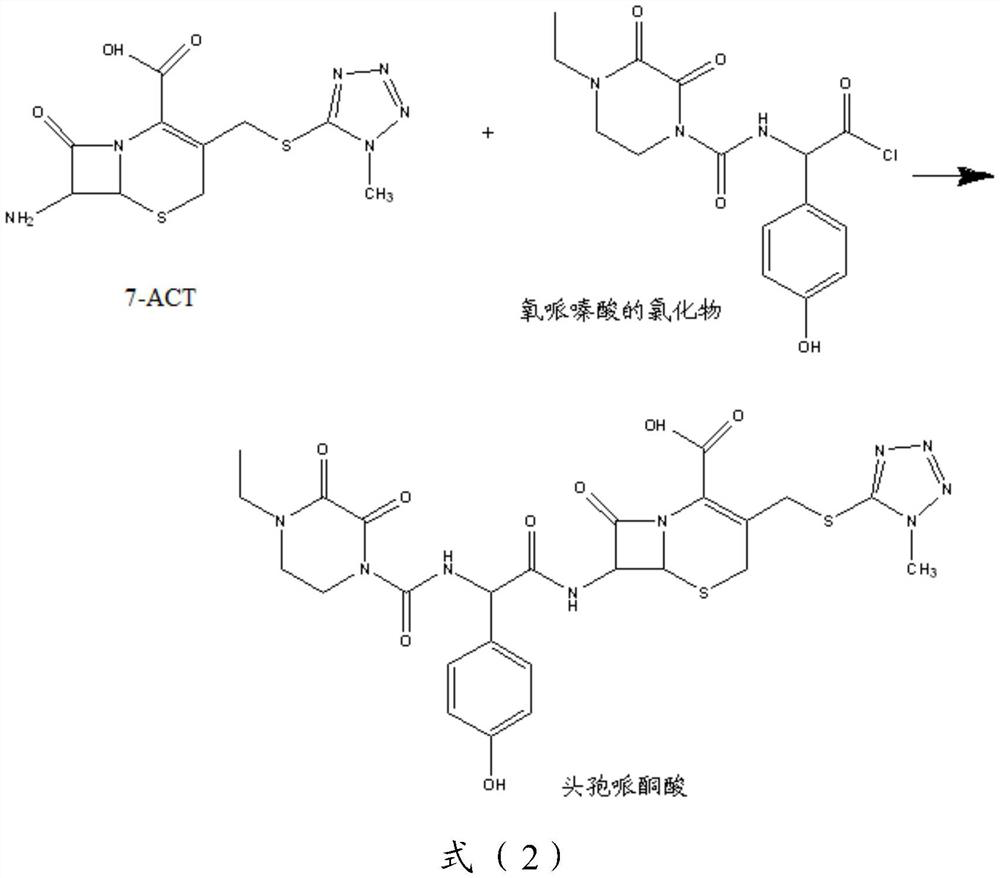
[0110] Step 2): Dissolve 353.8g of oxypiperazine acid chloride (1.0mol, M=353.76) in 1100mL of N,N-dimethylformamide, stir and cool to -20°C. Dissolve the prepared 7-ACT in 1100mL N,N-dimethylformamide, add 34mL trimethylchlorosilane under ice-bath cooling to remove the mo...
Embodiment 2
[0115] The synthesis of embodiment 2 cefoperazone sodium
[0116] The same as the synthesis process of Example 1, the only difference is: in step 1), in step 1), MTT 139.4g (1.20mol), that is, the molar ratio of 7-ACA and MTT is 1:1.20.
Embodiment 3
[0117] The synthesis of embodiment 3 cefoperazone sodium
[0118] The same as the synthesis process of Example 1, the only difference is: in step 1), in step 1), MTT 151g (1.30mol), that is, the molar ratio of 7-ACA and MTT is 1:1.30.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com