Method for synthesizing phthalocyanine blue dye
A synthesis method and phthalocyanine blue technology, applied in the field of phthalocyanine blue dye synthesis, can solve the problems of increased energy consumption, high ammonia nitrogen waste water, complex synthesis process flow, etc., and achieve low consumption of raw materials, good product quality and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
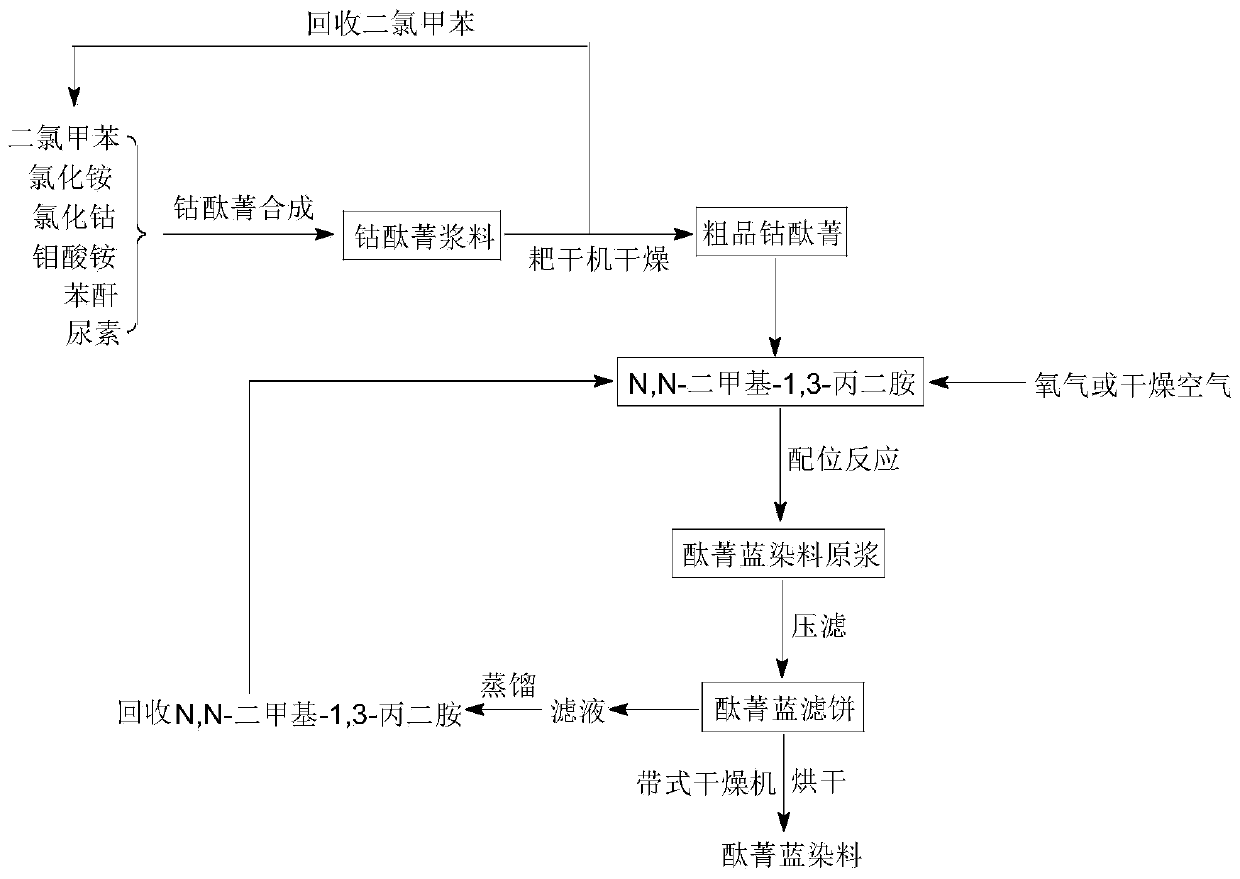
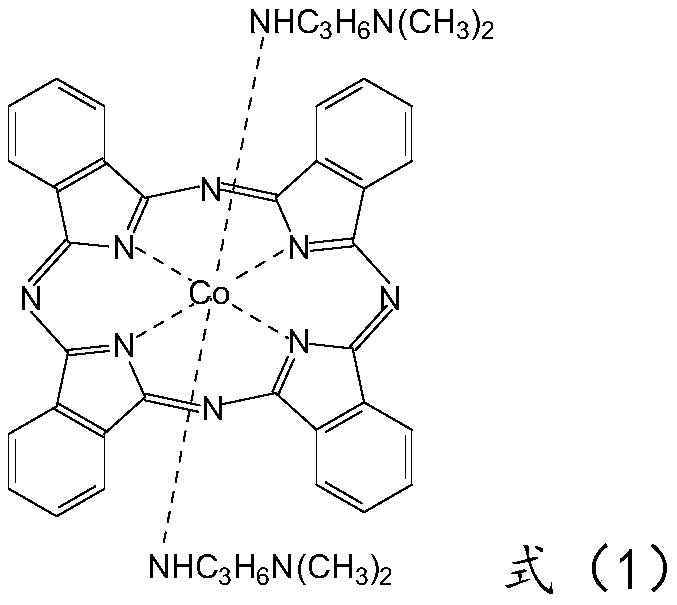
[0033] at 10m 3 Put 4000 kg of mixed dichlorotoluene (content ≥ 99.5%) into the cobalt phthalocyanine glass-lined reactor, start stirring, and put in 240 kg of weighed ammonium chloride (content ≥ 99.0%), cobalt chloride hexahydrate ( Content ≥ 24.0% (calculated as cobalt)) 335 kg, ammonium molybdate (content ≥ 99.0%) 19.5 kg, then heat up to about 160°C to remove the moisture in the raw materials. After the dehydration is basically complete, the temperature is naturally lowered to below 130°C, 840 kg of phthalic anhydride (content ≥ 99.0%) is fed from the feeding port of the reaction pot, and 1440 kg of urea (total nitrogen content ≥ 46.6%) is added after the temperature is lower than 110°C . After the temperature rises, the temperature is maintained at 192° C. to 197° C. and the reaction is kept for about 12 hours to complete the reaction. Then put the material into a rake dryer for vacuum rake dry distillation to recover mixed dichlorotoluene, and the rake dry temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com