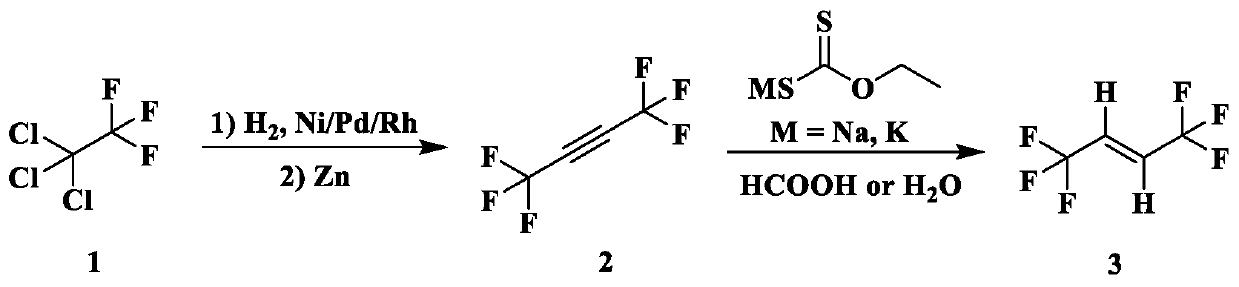
Preparation method of trans-1, 1, 1, 4, 4, 4-hexafluoro-2-butene
A technology of hexafluorobutyne and butene, which is applied in the field of organic fluorine chemical synthesis, can solve problems such as complex reactions and harsh conditions, achieve simple operation, mild preparation conditions, avoid high temperature conditions, highly toxic reagents and expensive metal catalysts The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The embodiment of this application discloses according to figure 1 The reaction scheme for the preparation of trans-1,1,1,4,4,4-hexafluoro-2-butene, figure 1 The reaction route is that trifluorotrichloroethane reacts with zinc under hydrogen and nickel catalyst conditions to produce 2-hexafluorobutyne, and 2-hexafluorobutyne reacts with ethyl xanthate potassium under formic acid or water conditions Reaction to prepare trans-1,1,1,4,4,4-hexafluoro-2-butene, the specific steps are as follows:
[0048] 1. Under room temperature, trifluorotrichloroethane (1.86g, 10mmol) and hydrogen with a partial pressure of 2MPa are passed into an autoclave filled with granular 5mol% activated carbon-supported nickel (25wt%) catalyst, and the temperature is gradually raised. Heat to 140°C for 1 hour, monitor the reaction by gas chromatography, cool to room temperature after the reaction, add a small amount of water to the collected product, filter and extract three times with dichloromet...
Embodiment 2- Embodiment 13
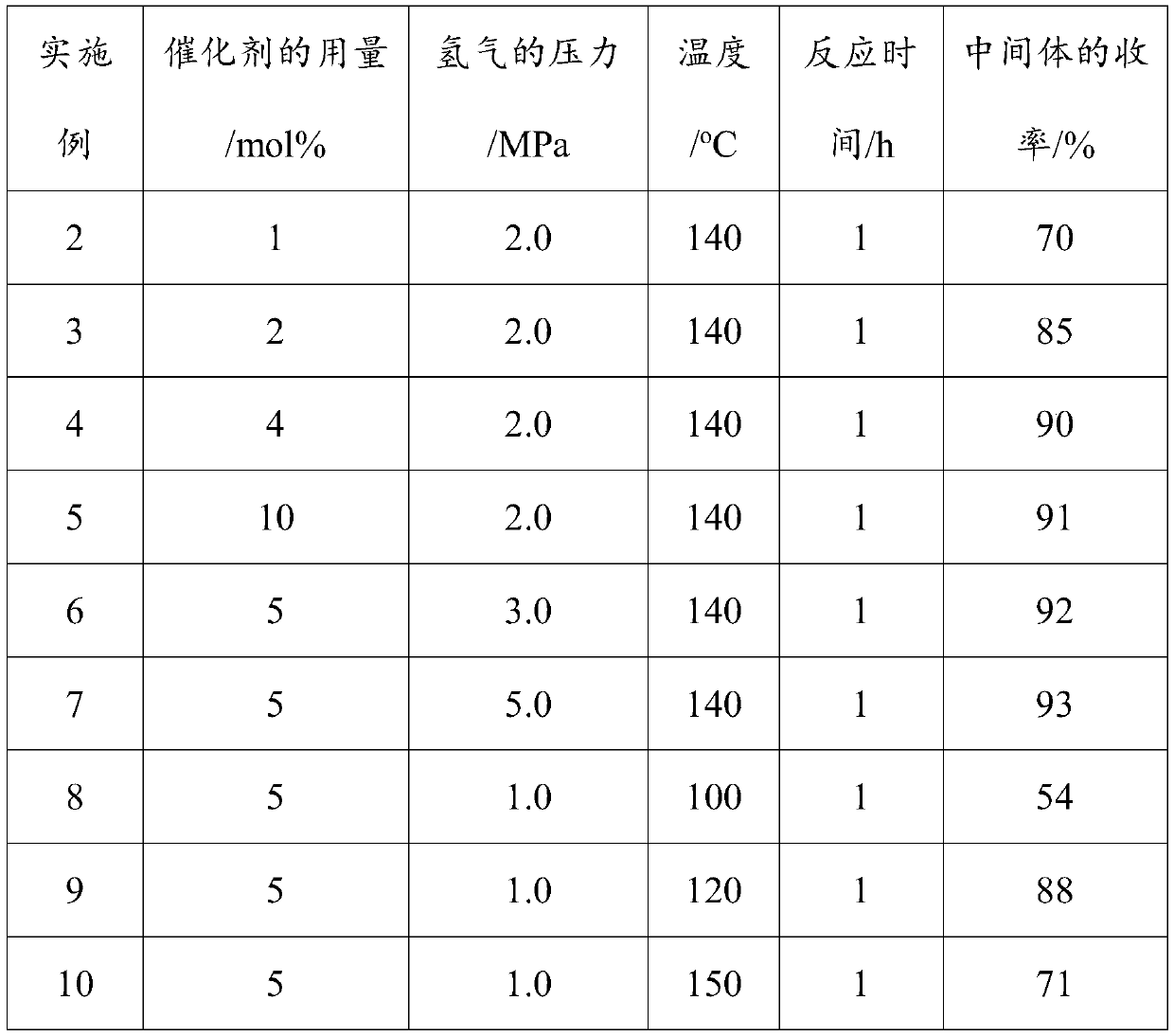
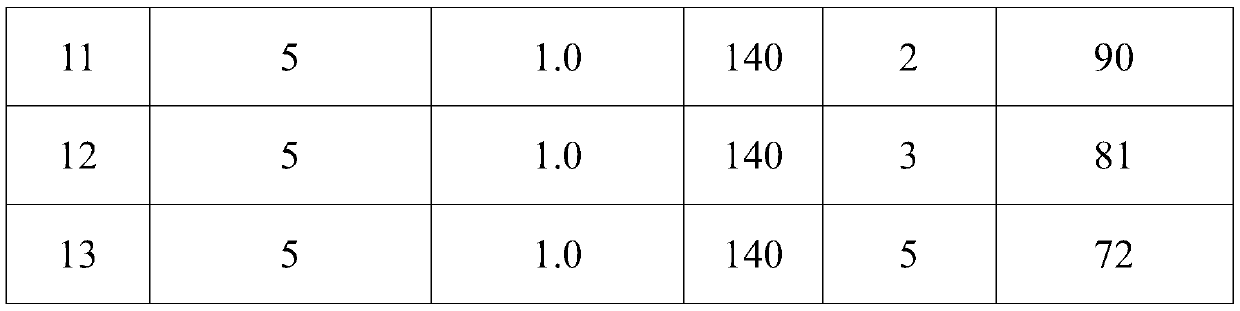
[0052] The embodiment of the present application provides the test that changes the consumption of the catalyst of the step 1 of embodiment 1, catalytic reaction temperature, catalytic reaction time and hydrogen pressure, then measure the yield of the pale yellow intermediate of embodiment 2-embodiment 13 ,Specific steps are as follows:
[0053] According to the method of step 1 among the embodiment 1, according to the consumption of gac-loaded nickel catalyst of table 1, the pressure that feeds hydrogen in step 1, the test that reaction time in step 1, reaction temperature in step 1 carries out, in different catalyst consumption, Different hydrogen pressures, different reaction times of trifluorotrichloroethane under hydrogen and activated carbon-supported nickel catalysts, and different reaction temperatures of trifluorotrichloroethane under hydrogen and activated carbon-supported nickel catalysts to prepare intermediates, determination step 1 The yield of the intermediate, ...
Embodiment 14- Embodiment 20
[0059] The embodiment of the present application provides a test of changing the amount of zinc powder in step 2 of embodiment 1, the reaction temperature of step 2, and the reaction time of step 2, and then measuring the 2-hexafluorobutyne of embodiment 14-embodiment 20 The yield, the specific steps are as follows:
[0060] According to the method of step 2 in embodiment 1, according to the solvent that adopts in the zinc powder consumption of table 2, step 2, reaction time in step 2, the test that reaction temperature carries out in step 2, in the consumption of different zinc powders, different kinds 2-hexafluorobutyne was prepared at different solvents, different reaction times and different reaction temperatures, and the yield of 2-hexafluorobutyne was measured. The results are shown in Table 2.
[0061] Table 2
[0062] Example Amount of zinc powder / equivalent solvent Reaction time / h Yield of 2-hexafluorobutyne / % 14 1 Acetic anhydride 10 80 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com