Diamine monomer containing isoindigo structure and black polyimide synthesized by diamine monomer
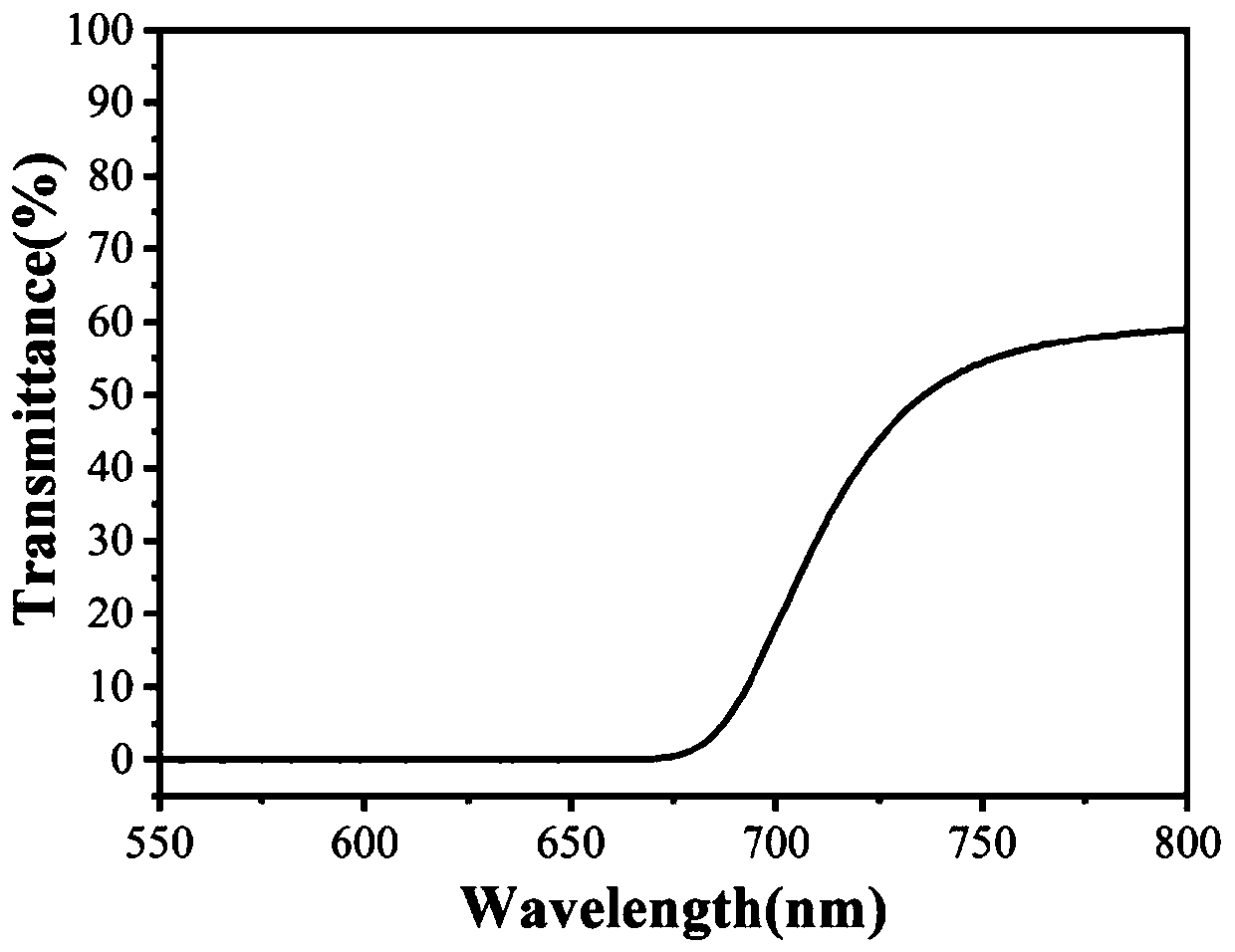
A black polyimide and diamine monomer technology, applied in the direction of organic chemistry, can solve the problems of strict coating process requirements, high production conditions, complex preparation process, etc., achieve excellent shading performance, easy synthesis, and efficient reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
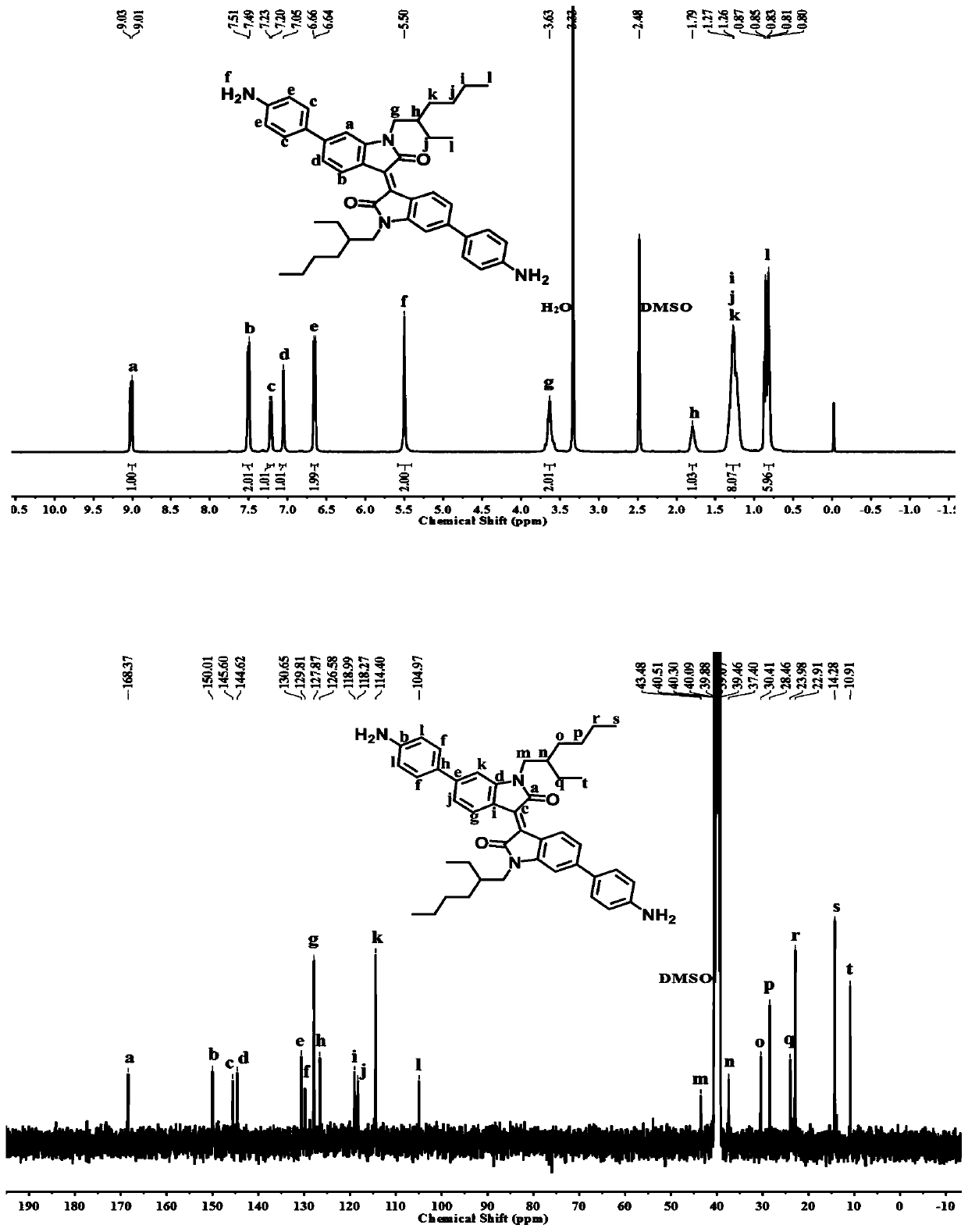
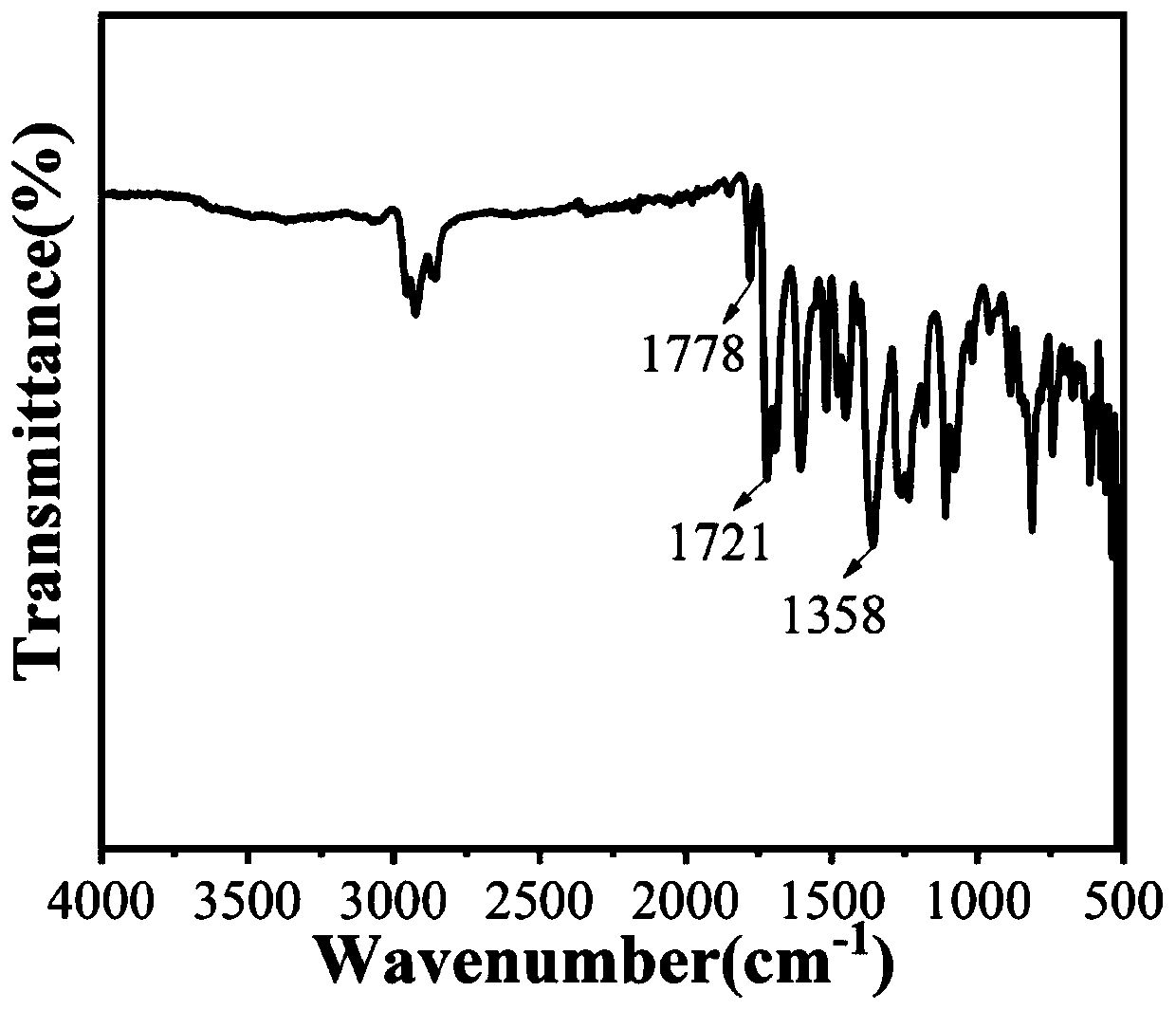
[0053] This example provides a (E) 6,6'-bis-(4-amino-phenyl)-1,1'-bis-(2-ethylhexyl)-[3,3'-dimethylene Synthesis method of ]-2,2'-diketone and black polyimide:
[0054] (1) (E) 6,6'-bis-(4-amino-phenyl)-1,1'-bis-(2-ethylhexyl)-[3,3'-dimethylhexyl]-2 , Synthesis of 2'-diketones:
[0055] ① Take a 250ml two-necked flask, weigh 6-bromo-indol-2-one (5g, 0.236mmol) and 6-bromoisatin (5.33g, 0.236mmol) with an analytical balance, add acetic acid (150mL) and Concentrated hydrochloric acid (37%) (1 mL) was refluxed at 120°C for 24 hours. After the reaction was completed, it was cooled to room temperature, the solvent was removed by suction filtration, the solid matter was washed with water, ethanol, and ethyl acetate in sequence, and dried in an oven overnight. After drying in vacuo, the reddish-brown product 1 was obtained.
[0056] ②Take a 250ml two-necked flask, weigh the product 1 (5g, 11.9mmol) and potassium carbonate (8.23g, 59.5mml) with an analytical balance, add DMF (80mL...
Embodiment 2
[0067] This example provides a (E) 6,6'-bis-(4-amino-phenyl)-1,1'-bis-(2-ethylhexyl)-[3,3'-dimethylene Synthesis method of ]-2,2'-diketone and black polyimide:
[0068] (1) (E) 6,6'-bis-(4-amino-phenyl)-1,1'-bis-(2-ethylhexyl)-[3,3'-dimethylhexyl]-2 , Synthesis of 2'-diketones:
[0069] ① Take a 250ml two-necked flask, weigh 6-bromo-indol-2-one (5g, 0.236mmol) and 6-bromoisatin (5.33g, 0.236mmol) with an analytical balance, add acetic acid (150mL) and Concentrated hydrochloric acid (37%) (1 mL) was refluxed at 120°C for 24 hours. After the reaction was completed, it was cooled to room temperature, the solvent was removed by suction filtration, the solid matter was washed with water, ethanol, and ethyl acetate in sequence, and dried in an oven overnight. After drying in vacuo, the reddish-brown product 1 was obtained.
[0070] ②Take a 250ml two-necked flask, weigh the product 1 (5g, 11.9mmol) and potassium carbonate (8.23g, 59.5mml) with an analytical balance, add DMF (80mL...
Embodiment 3
[0078] (1) (E) 6,6'-bis-(4-amino-phenyl)-1,1'-bis-(2-ethylhexyl)-[3,3'-dimethylhexyl]-2 , Synthesis of 2'-diketones:
[0079] ① Get a 250ml two-necked flask, weigh 6-bromo-indol-2-one (5g, 0.236mmol) and 6-bromoisatin (5.33g, 0.236mmol) with an analytical balance, add acetic acid (150mL) and Concentrated hydrochloric acid (37%) (1 mL) was refluxed at 120°C for 24 hours. After the reaction was completed, it was cooled to room temperature, the solvent was removed by suction filtration, the solid matter was washed with water, ethanol, and ethyl acetate in sequence, and dried in an oven overnight. After drying in vacuo, the reddish-brown product 1 was obtained.
[0080] ②Take a 250ml two-necked flask, weigh the product 1 (5g, 11.9mmol) and potassium carbonate (8.23g, 59.5mml) with an analytical balance, add DMF (80mL) to vacuumize, and pass nitrogen 3 times; stir at 80°C After 0.5-3h, 1-bromo-2-ethylhexane (4.7ml, 26.2mmol) was injected into the septum under the protection of nit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com