Synthesis method of enzyme-catalyzed (1S, 5R)-bicyclolactone
A synthesis method and bicyclic lactone technology are applied in the field of biopharmaceuticals, which can solve the problems of small production scale and troublesome post-processing, and achieve the effects of improving separation conditions and avoiding the use of chemical catalytic reagents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
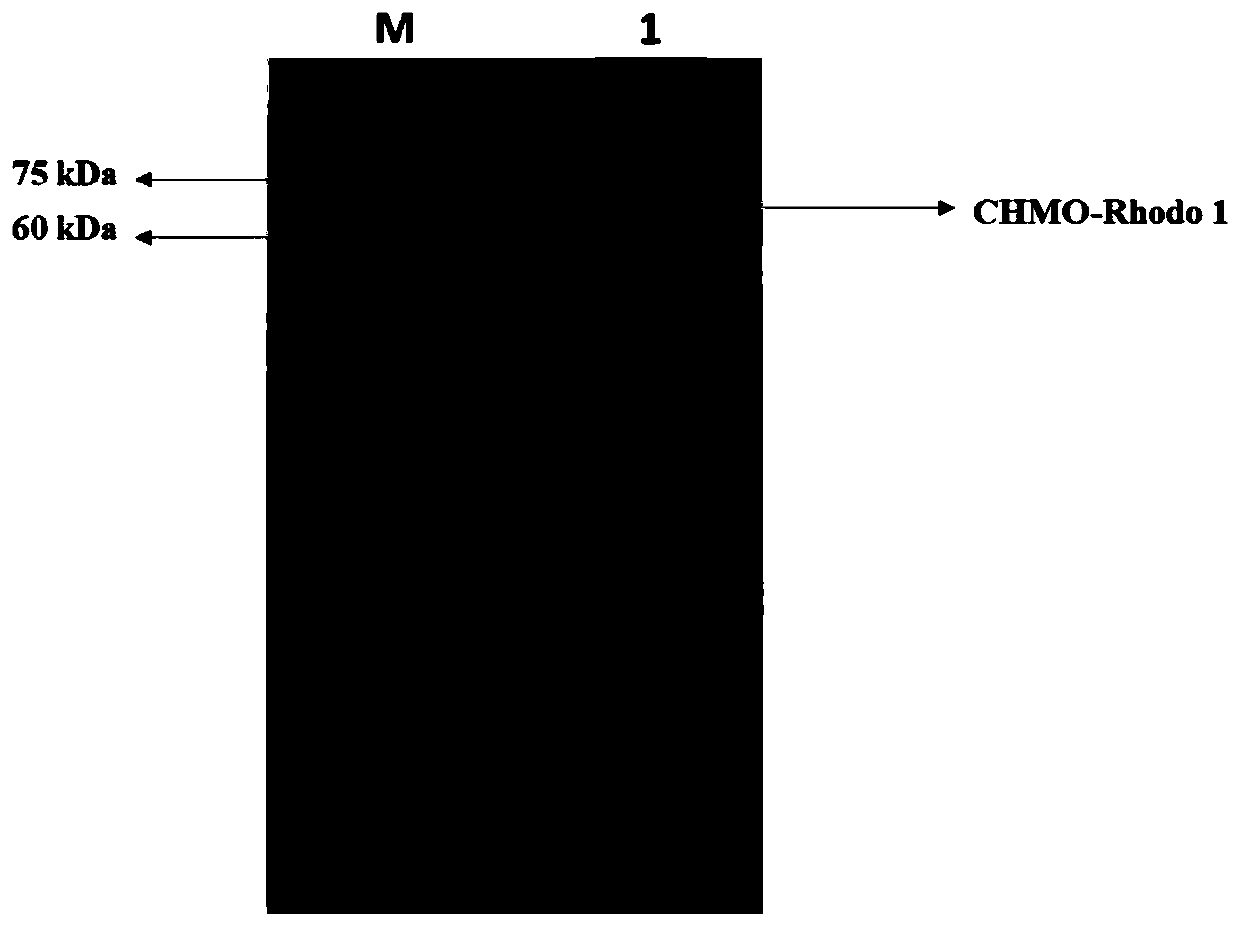
[0042] Embodiment 1, construction and induced expression of genetically engineered bacteria
[0043] Transform the expression host BL21(DE3) with the plasmid CHMO-Rhodo1. Inoculate BL21(DE3)-CHMO-Rhodo1 into a 5mL liquid LB test tube culture medium containing kanamycin resistance, and activate it on a shaker at 37°C for 8 hours , the activated culture was transferred to the liquid LB shake flask medium containing kanamycin resistance according to 1% transfer amount, and the fermentation medium was shaken at constant temperature for 3 hours, and the culture temperature was 37°C, 200rpm. When the cell concentration grows to OD 600 When = 1.2, add 0.1mM IPTG (final concentration), induce at 18°C for 18h, collect cells by centrifugation at 8500rpm for 10min, resuspend with pH7.5, 250mM sodium phosphate buffer, break with 45% ultrasonic power for 30min, and centrifuge to obtain The cell supernatant containing CHMO-Rhodo1 was stored in a 4-degree refrigerator.
Embodiment 2
[0044] Embodiment 2, reaction process monitoring
[0045] Adopt GC detection method to measure racemic substitution bicyclo [3.2.0]-hept-2-en-6-ketone (II) to (1 S , 5 R )-transformation of bicyclic lactone (I). Take 100 L of the reaction solution at different reaction time points, add an equal volume of ethyl acetate solution, mix well, centrifuge to take the upper ethyl acetate layer, and filter it with a 0.3M filter membrane for detection. Chromatographic conditions: chromatographic column DB-624 (0.32mm × 30m), detector: DAD detector, sample volume: 1 L, heating program: initial temperature: 80°C, 80-130°C: heating rate 10°C / min, 130-230°C: heating rate 20°C / min, hold for 2min. The retention time of racemic substituted bicyclo[3.2.0]-hept-2-en-6-one (II) is 7.8min, and its structure identification 1 H NMR as Figure 5 As shown, (1 S , 5 R )-bicyclic lactone (I) has a retention time of 11.02min, and its structure identification 1 H NMR as Image 6 shown. The absol...
Embodiment 3
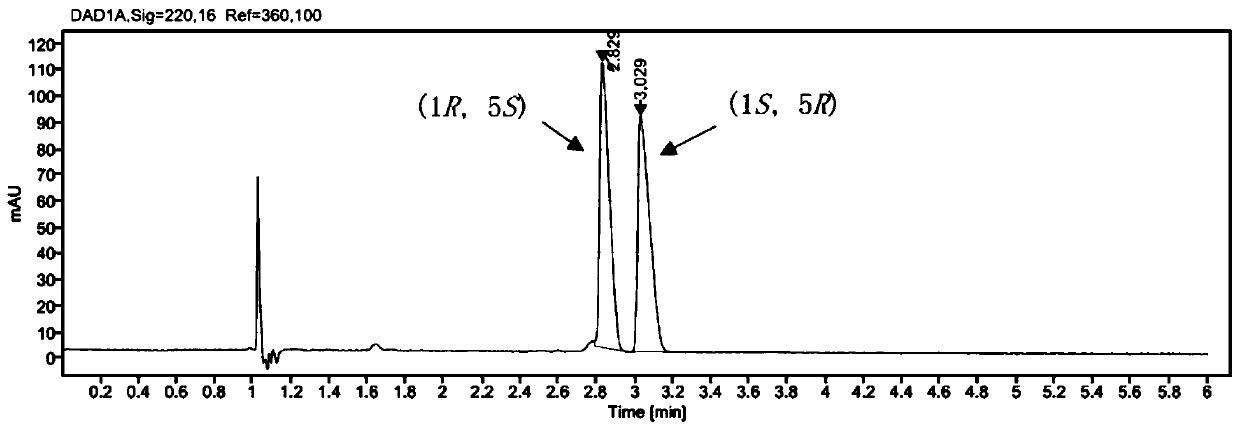
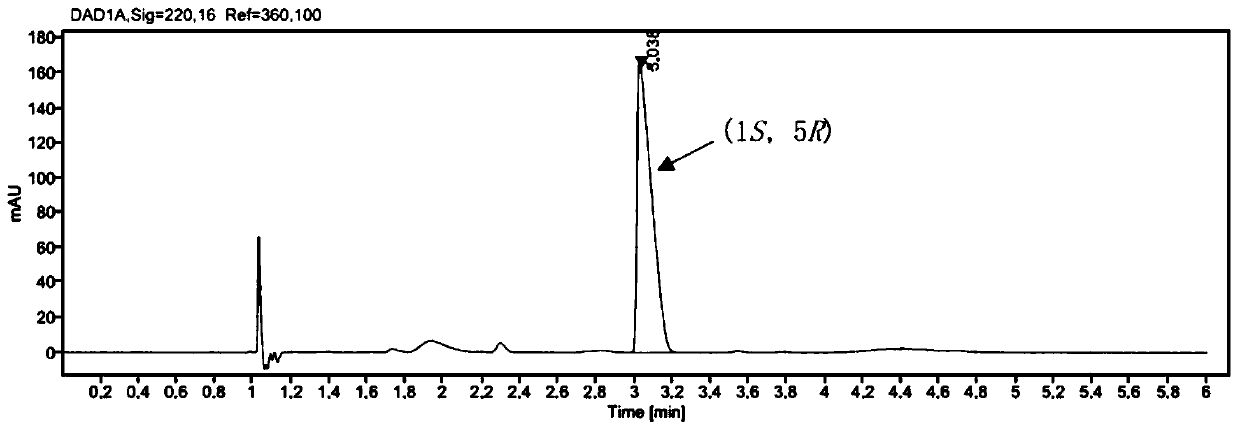
[0046] Embodiment 3, the mensuration of product ee value
[0047] target product (1 S , 5 R )-The determination of the ee value of bicyclic lactone (I) adopts the method of chiral SFC to measure. Sample treatment: After the post-treatment product was dissolved in chromatographic grade anhydrous methanol, it was detected by SFC.
[0048] SFC analysis conditions: Chromatographic column: Chiralpak IF (4.6mm × 250mm), mobile phase: CO 2 / MeOH=95:5, flow rate: 3mL / min, injection volume: 5 L, detection wavelength: 220nM, the HPLC spectrum of the racemic product is as follows figure 2 As shown, (1R, 5 S )-7, the retention time of 7-dichlorobicyclic lactone is 2.82min, (1 S , 5 R )-7,7-dichlorobicyclic lactone (I) has a retention time of 3.029min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com