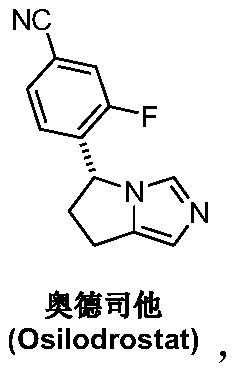
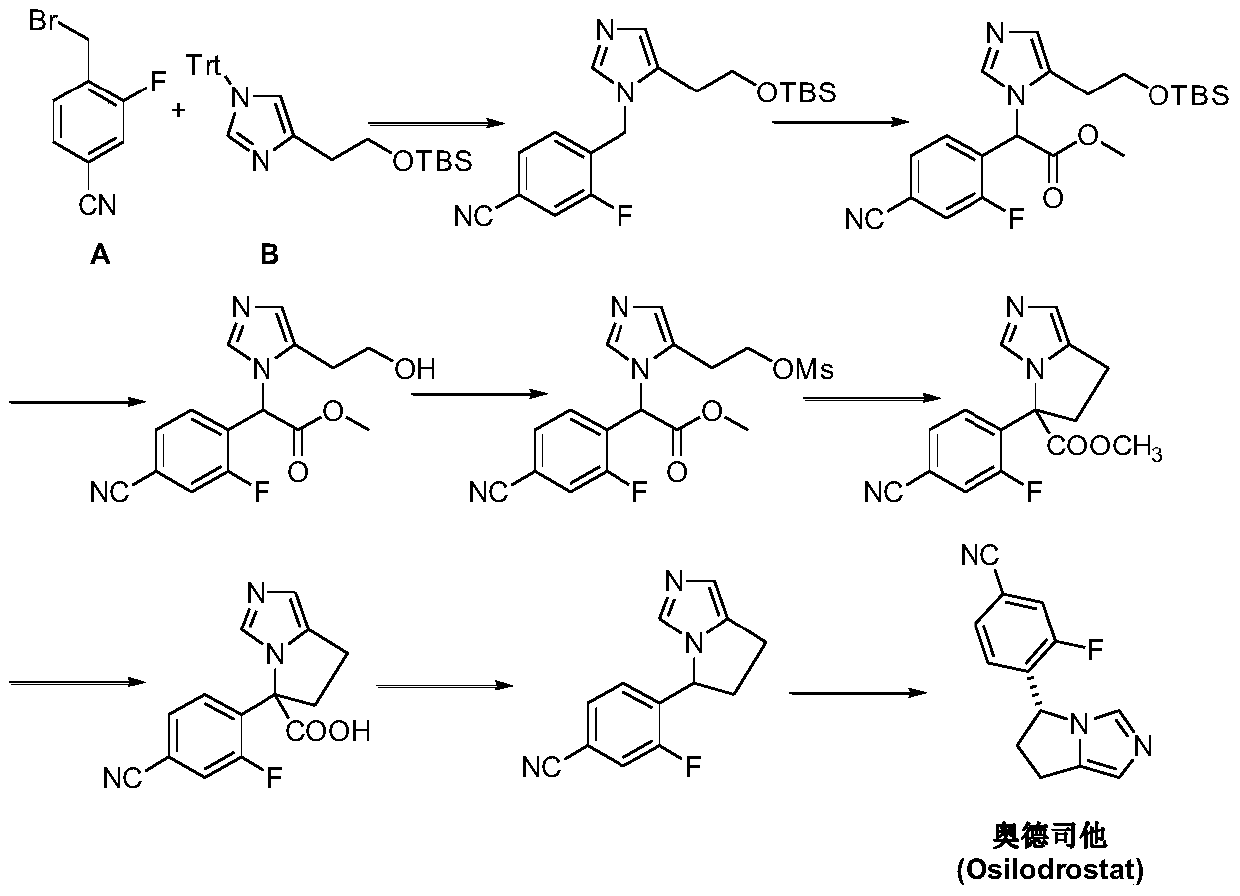
Preparation method of osilodrostat
A technology of odes and benzonitrile, applied in the field of preparation of Cushing's syndrome drug odes, can solve the problems of low total yield, many reaction steps, restricting industrial production and the like, and achieves favorable conditions for industrial production and conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Under nitrogen protection, 6-(6-trifluoromethyl-2-yl)-1,3,5-triazine-2,4-dione (II) (18g, 0.1mol), 1 , 3-dihydroxyacetone dimer (18g, 0.1mol), potassium thiocyanate (9.7g, 0.1mol), solvent acetonitrile 100mL and water 50mL, keep the temperature at 5-10°C, and react for 10 hours. The temperature was lowered to 0°C, 30% hydrogen peroxide (31 mL, 0.3 mol) was added, and the reaction was continued to stir for 2 hours, and the reaction was monitored by TLC to complete. The reaction was quenched with saturated sodium sulfite solution and adjusted to pH = 8-10 with solid sodium carbonate. Ethyl acetate was added to extract 3 times, the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was recovered by distillation under reduced pressure, and the oily substance 4-[(1R)-(2-hydroxymethyl-1H-imidazol-1-yl)- 2-Hydroxyethyl]-3-fluoro-benzonitrile (III) 18.5g, yield 70.9%, EI-MS m / z: 262[M+H] + .
Embodiment 2
[0039] Add 4-[(1R)-(2-hydroxymethyl-1H-imidazol-1-yl)-2-hydroxyethyl]-3-fluoro-benzonitrile (III) (13g, 50mmol) into the three-necked flask, Manganese dioxide (13 g, 150 mmol) and dioxane 150 mL. The temperature was raised to 80°C, and the reaction was carried out for 8 hours, and the reaction was completed by TLC monitoring. Remove the solid by filtration with celite, and concentrate under reduced pressure to give a yellow oily substance 4-[(1R)-(2-formyl-1H-imidazol-1-yl)-2-hydroxyethyl]-3-fluoro-benzene Nitrile (IV) 10.4g, yield 80.3%, EI-MS m / z: 260[M+H] + .
Embodiment 3
[0041]Add 30mL of concentrated sulfuric acid and 15mL of water to the reaction flask under ice-cooling, and add 4-[(1R)-(2-formyl-1H-imidazol-1-yl)-2-hydroxyethyl]-3 - Fluoro-benzonitrile (IV) (7.8 g, 30 mmol) and sodium bromide (3.7 g, 36 mmol) and dioxane 30 mL. The temperature was raised to 90° C., and the reaction was carried out for 5 hours, and the reaction was monitored by TLC to complete. Stand still, take the supernatant, extract three times with ethyl acetate, combine the organic phases, wash with water, saturated sodium bicarbonate solution and water successively, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. 8.8 g of yellow-red liquid 4-[(1R)-(2-formyl-1H-imidazol-1-yl)-2-bromo-ethyl]-3-fluoro-benzonitrile (V) was obtained, and the yield was 91.1 %, EI-MS m / z:323[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com