Asymmetrically substituted 1, 3, 5-triazine compound as well as preparation method and application thereof
A technology of ester compounds and compounds, which is applied in the field of biocatalytic synthesis, can solve the problems of low yield, unfriendly environment, and toxic catalysts, and achieve the effects of high yield, reduced catalyst consumption, and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] A specific synthetic route of an asymmetrically substituted 1,3,5-triazine compound is:
[0087]
[0088] Its concrete synthetic steps are as follows:
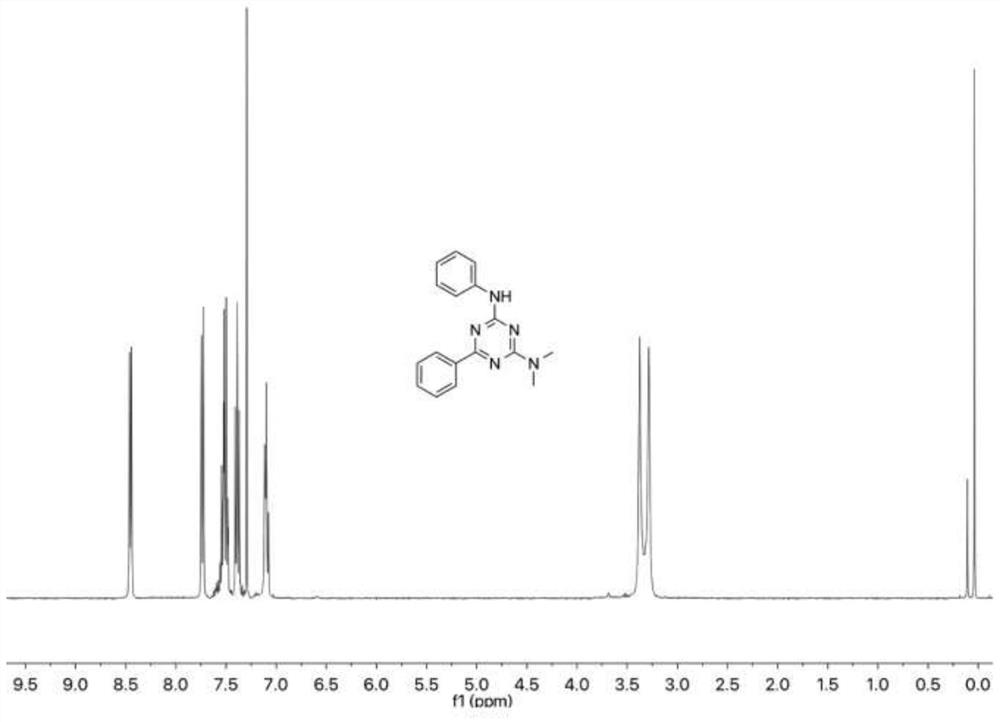
[0089] Add 13.5 mg of phenylisothiocyanate and 11.5 mg of tetramethylguanidine to 1 mL of dimethyl sulfoxide, then add 15.6 mg of benzamidine hydrochloride and rabbit hemoglobin (0.5 mol), and slowly add three times the amount tert-butyl hydroperoxide and reacted at 25°C (normal temperature) for ten minutes, and then detected the reaction progress by thin-layer chromatography; then added water to quench the reaction, extracted with ethyl acetate and added anhydrous sodium sulfate to dry , concentrated, followed by further purification by silica gel column chromatography (ethyl acetate / hexane) to obtain N 2 ,N 2 -Dimethyl-N 4 ,6-diphenyl-2,4-diamino-1,3,5-triazine 26.5 mg, yield 91%, white solid.
[0090] In this embodiment, the product is subjected to nuclear magnetic detection and analysis. For specific nuclear ...
Embodiment 2
[0093] A specific synthetic route of an asymmetrically substituted 1,3,5-triazine compound is:
[0094]
[0095] Its concrete synthetic steps are as follows:
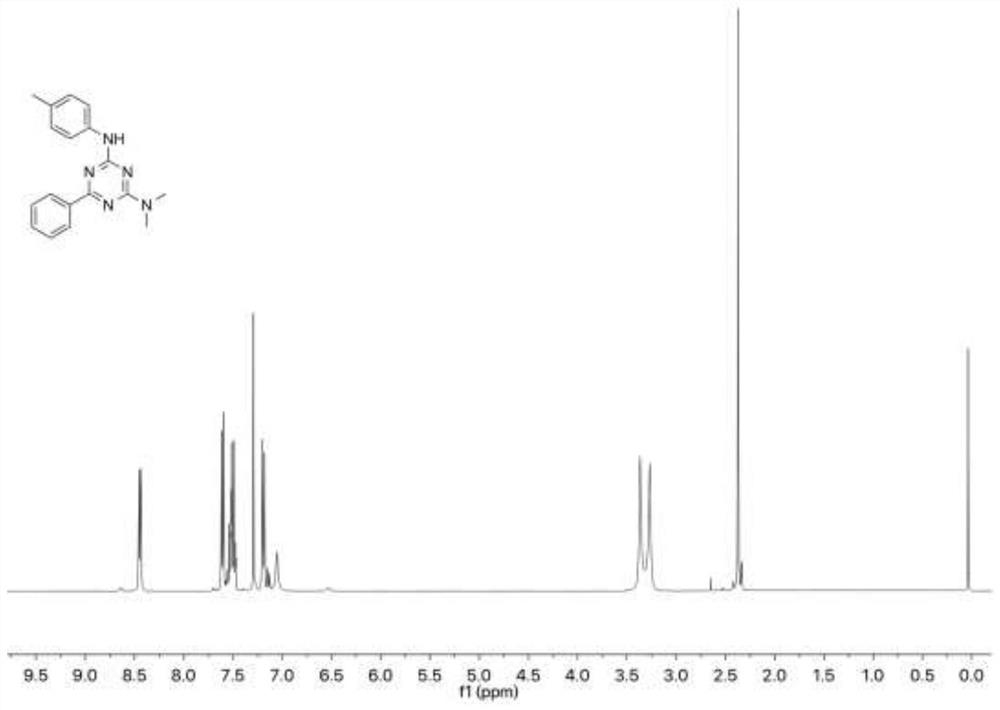
[0096] Add 14.9 mg of 4-methylphenyl isothiocyanate and 11.5 mg of tetramethylguanidine into 1 mL of dimethyl sulfoxide, then add 15.6 mg of benzamidine hydrochloride and rabbit hemoglobin (0.5 mol), slowly drop After adding three times the amount of tert-butyl hydroperoxide, react at 25°C (normal temperature) for ten minutes, and then use thin-layer chromatography to detect the reaction progress; then add water to quench the reaction, extract with ethyl acetate and add anhydrous After drying over sodium sulfate, concentration, followed by further purification by silica gel column chromatography (ethyl acetate / hexane) to obtain N 2 ,N 2 -Dimethyl-N 4 -(p-Tolyl)-6-phenyl-2,4-diamino-1,3,5-triazine 27.5 mg, yield 90%, white solid.
[0097] In this example, the product is subjected to nuclear magnetic detection and an...
Embodiment 3
[0100] A specific synthetic route of an asymmetrically substituted 1,3,5-triazine compound is:
[0101]
[0102] Its concrete synthetic steps are as follows:
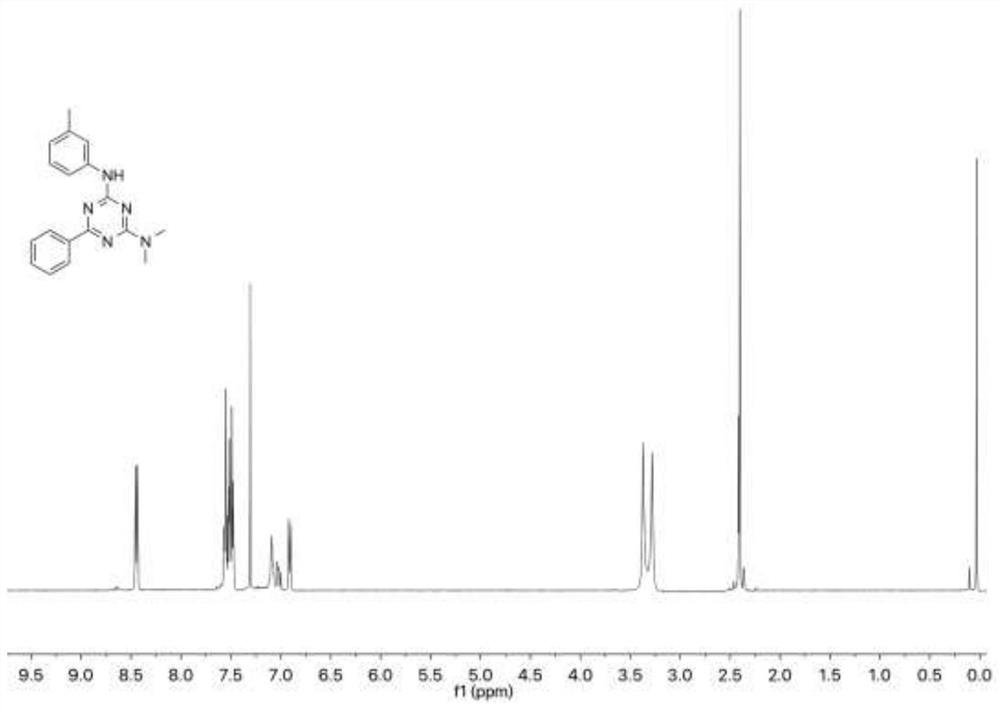
[0103] Add 14.9 mg of 3-methylphenyl isothiocyanate and 11.5 mg of tetramethylguanidine into 1 mL of dimethyl sulfoxide, then add 15.6 mg of benzamidine hydrochloride and rabbit hemoglobin (0.5 mol), slowly drop After adding three times the amount of tert-butyl hydroperoxide, react at 25°C (normal temperature) for ten minutes, and then use thin-layer chromatography to detect the reaction progress; then add water to quench the reaction, extract with ethyl acetate and add anhydrous After drying over sodium sulfate, concentration, followed by further purification by silica gel column chromatography (ethyl acetate / hexane) to obtain N 2 ,N 2 -Dimethyl-N 4 -(m-Tolyl)-6-phenyl-2,4-diamino-1,3,5-triazine 26.8mg, yield 88%, white solid.
[0104] In this example, the product is subjected to nuclear magnetic detection and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com