Abietane compound with C-ring combined lactone ring novel skeleton as well as preparation method and application of abietane compound
A technology of abietane and lactone rings, which is applied in the field of abietane compounds and their preparation, can solve the problems that are not commonly used, and the C ring is difficult to transform, and achieve good protective effect, good tumor suppression effect, and reduce the scope of cerebral infarction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
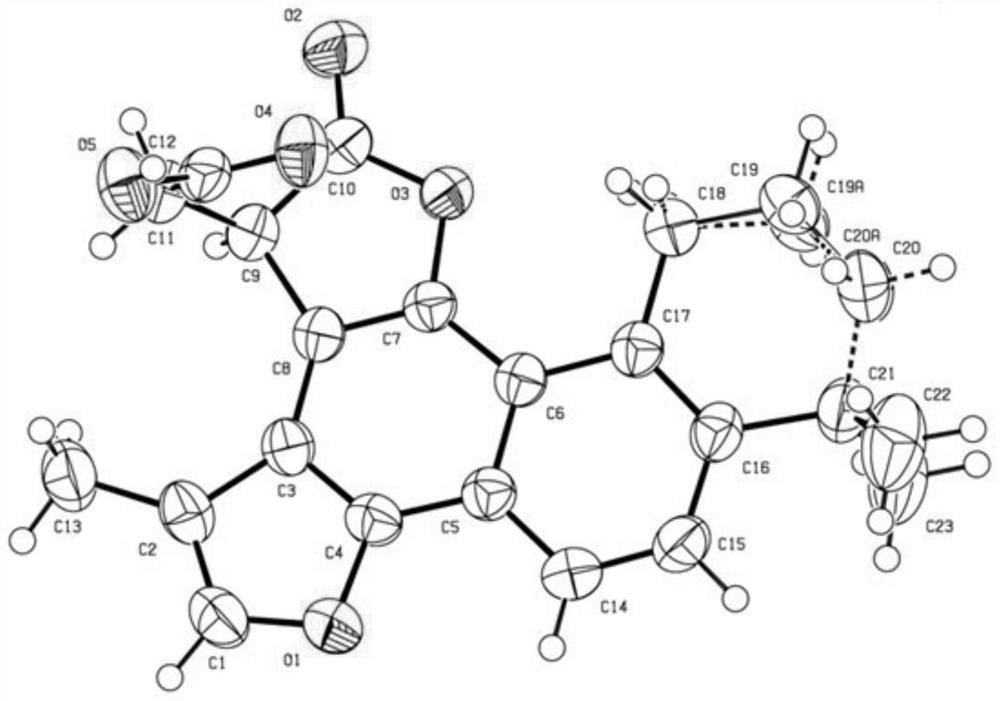
[0057] Weigh 10g (34mmol) of tanshinone IIA raw material in a reaction flask, add 100ml of tetrahydrofuran, add 3.4g (34mmol) of succinic anhydride, 9.5g of DMAP, and 6.6g of zinc powder while stirring, heat to reflux under nitrogen protection, and stir for 3 hours. TLC monitored the completion of the reaction. The zinc powder was removed by filtration, the filtrate was evaporated to dryness, recrystallized with methanol, and dried to obtain 9.6 g of the product (compound 1), with a yield of 75%. Its chemical structure is confirmed by spectral analysis and X-single crystal diffraction as follows:
[0058]
[0059] The spectral data of compound 1 are as follows:
[0060] ESI–MS: m / z=379.6[M+H] + .
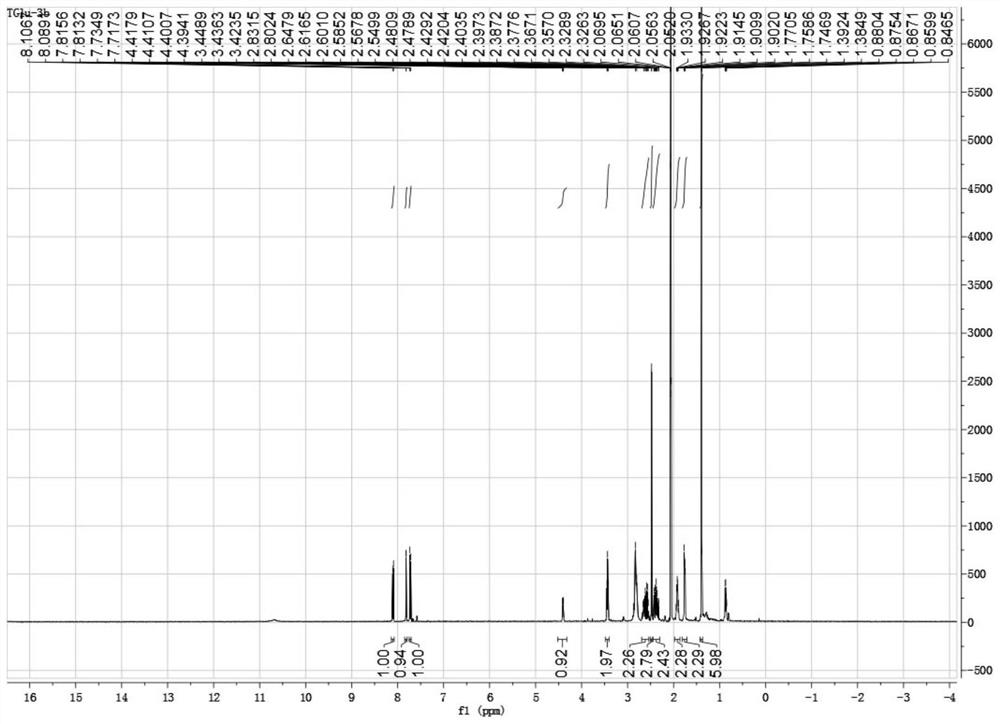
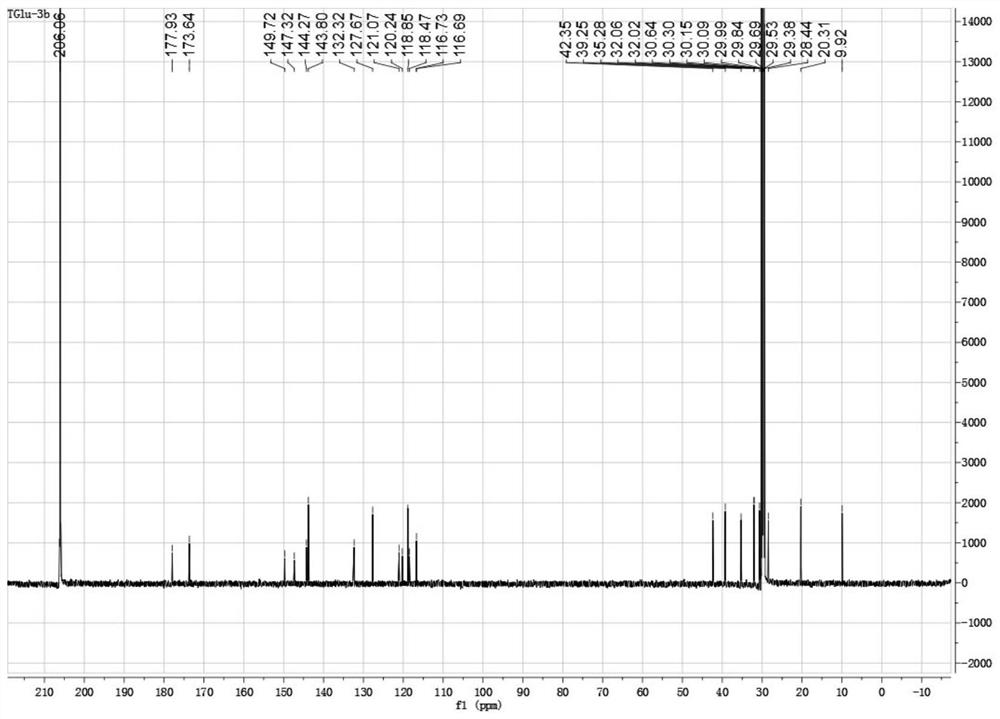
[0061] 1 H NMR (600MHz, DMSO-d 6 )δ12.43(s,1H),8.03(d,J=8.8Hz,1H),7.91(d,J=1.2Hz,1H),7.69(d,J=8.8Hz,1H),4.54(t, J=4.6Hz,1H),3.38–3.31(m,2H), 3.25(dd,J=17.2,5.6Hz,1H),3.14(dd,J=17.2,5.6Hz,1H),2.35(d,J =1.2Hz,3H), 1.92–1.79(m,2H),1.77–1.64(m,2H),1.35(s,3H),1.34(s,3H); 13 C ...
Embodiment 2
[0063] Weigh 10g (34mmol) of Tanshinone IIA raw material in the reaction flask, add 100ml tetrahydrofuran,
[0064] While stirring, 3.88 g (34 mmol) of glutaric anhydride, 7.8 g of DMAP, and 5.9 g of iron powder were added, and heated to reflux under the protection of nitrogen. After stirring for 6 hours, the reaction was complete as monitored by TLC. The iron powder was removed by filtration, and the filtrate was evaporated to dryness, recrystallized with acetone, and dried to obtain 10.8 g of the product (compound 1), with a yield of 81%. Its chemical structure was confirmed by spectral analysis as follows:
[0065]
[0066] The spectral data of compound 2 are as follows:
[0067] ESI-MS: m / z=391.30[M-H] - .
[0068] 1 H NMR (500MHz, Acetone-d 6 )δ8.09(d, J=8.8Hz, 1H), 7.80(d, J=1.2Hz, 1H), 7.72(d, J=8.8Hz, 1H), 4.39(dd, J=8.5, 3.5Hz, 1H), 3.43(t, J=6.4Hz, 2H), 2.47(d, J=1.0Hz, 3H), 2.44–2.31(m, 3H), 1.94–1.87(m, 2H), 1.75(dd,J =12.1, 6.2Hz, 2H), 1.38(d, J=3.7Hz, 6...
Embodiment 3
[0070] Weigh 1.0g (2.55mmol) of the raw material of compound 2 into a reaction flask, add 30ml of dichloromethane, add 1.2g of DMAP, 0.6g of DCC, and 0.2ml of triethylamine while stirring, heat to reflux under nitrogen protection, and stir for 5 hours , TLC monitors that the reaction is complete. The reaction was quenched with ice water, extracted with dichloromethane (20ml×3), combined, the organic phase was concentrated, and separated by silica gel column to obtain 0.59 g of the product (compound 3), with a yield of 59%. Its chemical structure was confirmed by spectral analysis as follows:
[0071]
[0072] The spectral data of compound 3 are as follows:
[0073] ESI-MS: m / z=391.28]M-H] - .
[0074] 1 H NMR (500MHz, Acetone-d 6 )δ10.66(1H,br.s),8.11(d,J=8.7Hz,1H),7.79(s,1H),7.63(d,J=8.7Hz,1H),4.55(dd,J=9.7 ,2.9Hz,1H),3.42–3.39(m,1H),3.26 (d,J=16.3Hz,1H),2.62–2.47(m,2H),2.42–2.36(m,4H),2.02–1.95( m,2H),1.39(s,3H),1.36(s,3H); 13 C NMR (125MHz, Acetone-d 6 )δ178.19,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com