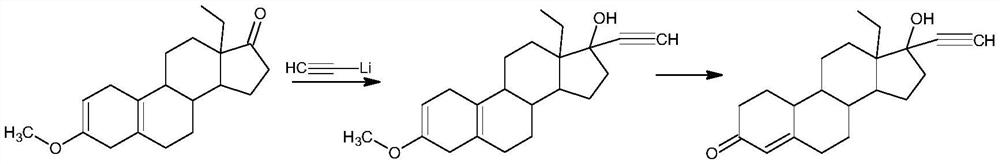
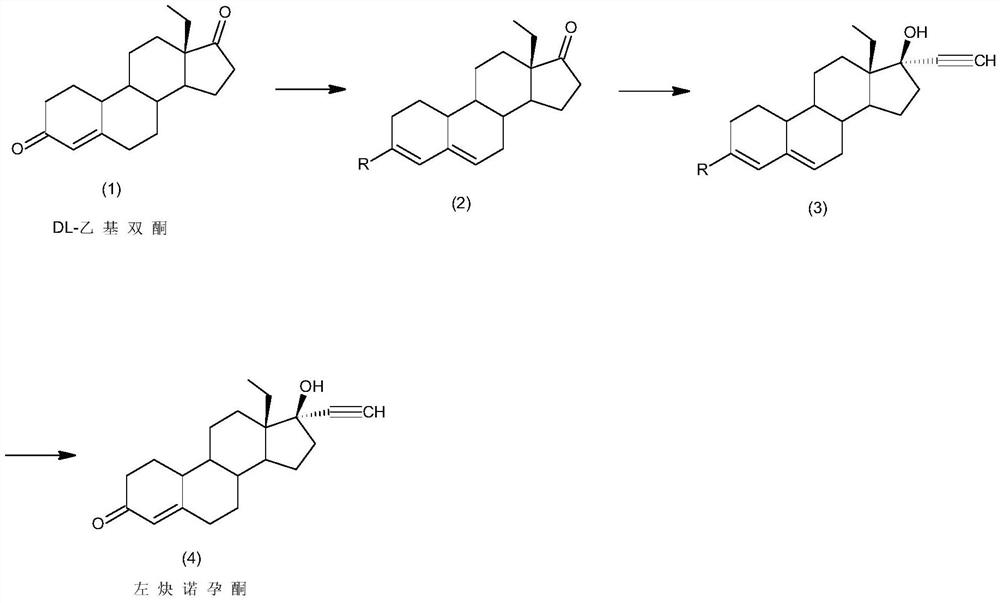
Synthesis method of levonorgestrel
A technology of levonorgestrel and a synthesis method is applied in the field of drug preparation, can solve the problems of unsuitability for industrialized safe production, dangerous large lithium ammonia reagent, long reaction route and the like, achieves good market prospect, low operating cost, Easy route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of synthetic method of embodiment 1 levonorgestrel, comprises the steps:
[0025] 1) Protection reaction: Add 50gDL-ethyl diketone (1) to 1000ml methanol, add 50ml trimethyl orthoformate, 0.5g pyridinium hydrobromide, control the temperature at 60°C and stir the reaction. After the reaction, add 0.5ml Triethylamine, cooled to -10°C, filtered and dried to obtain 50g of intermediate 2;
[0026] 2) Alkynylation reaction: Add 50 g of intermediate (2) obtained in step 1) to 500 ml of acetone, add 100 g of potassium tert-butoxide, pass through acetylene, and control the reaction at -20 ° C. After the reaction, add 10% Aqueous hydrochloric acid was neutralized to neutral, concentrated, added water for water analysis, and filtered to obtain 53g of intermediate (3);
[0027] 3) Hydrolysis reaction: 53g of intermediate (3) obtained in step 2) was added to 530ml of tetrahydrofuran, 100ml of 20% hydrochloric acid aqueous solution was added, and the reaction was stirred at 5...
Embodiment 2
[0028] A kind of synthetic method of embodiment 2 levonorgestrel, comprises the steps:
[0029] 1) Protection reaction: Add 50gDL-ethyl diketone (1) to 50ml ethanol, add 250ml triethyl orthoformate, 2g pyridine hydrochloride, control the temperature at 40°C and stir the reaction. After the reaction, add 2ml triethylamine , cooled to 5°C, filtered and dried to obtain 51g of intermediate 2;
[0030] 2) Alkynylation reaction: 51g of intermediate (2) obtained in step 1) was added to 250ml of tetrahydrofuran, 26g of potassium isobutoxide was added, acetylene was passed through, and the reaction was stirred at 10°C. After the reaction, 20% sulfuric acid was added The aqueous solution was neutralized to neutral, concentrated, added water for water analysis, filtered to obtain the crude product, and the crude product was obtained with ethanol to obtain 53g of intermediate (3);
[0031] 3) Hydrolysis reaction: 53g of intermediate (3) obtained in step 2) was added to 1500ml of acetone,...
Embodiment 3
[0032] A kind of synthetic method of levonorgestrel of embodiment 3 comprises the steps:
[0033] 1) Protection reaction: Add 50gDL-ethyl diketone (1) to 200ml pyrrolidine, add 100ml triethyl orthoformate, 10g p-toluenesulfonic acid, control the temperature at 20°C and stir the reaction. After the reaction is over, add 10ml pyridine, Cool down to -5°C, filter, and dry to obtain 55g of intermediate 2;
[0034] 2) Alkynylation reaction: 55g of the intermediate (2) obtained in step 1) was added to 1500ml of toluene, 250g of sodium ethylate was added, acetylene was passed through, and the reaction was stirred at 40°C. After the reaction was completed, it was added to 30% acetic acid aqueous solution and to neutrality, concentrated, added water for water analysis, filtered to obtain 57g intermediate (3);
[0035] 3) Hydrolysis reaction: 57g of intermediate (3) obtained in step 2) was added to 300ml of methyl tetrahydrofuran, 29ml of 30% aqueous sulfuric acid was added, and the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com